429120
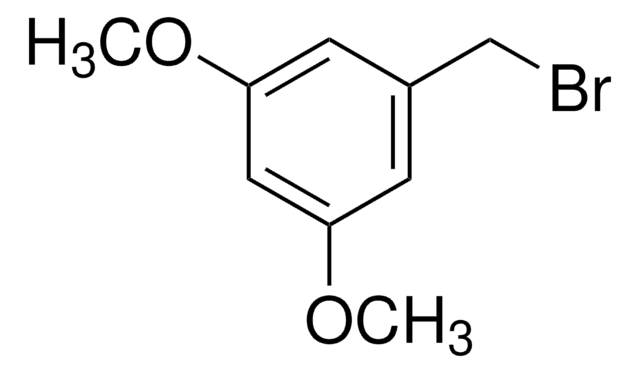
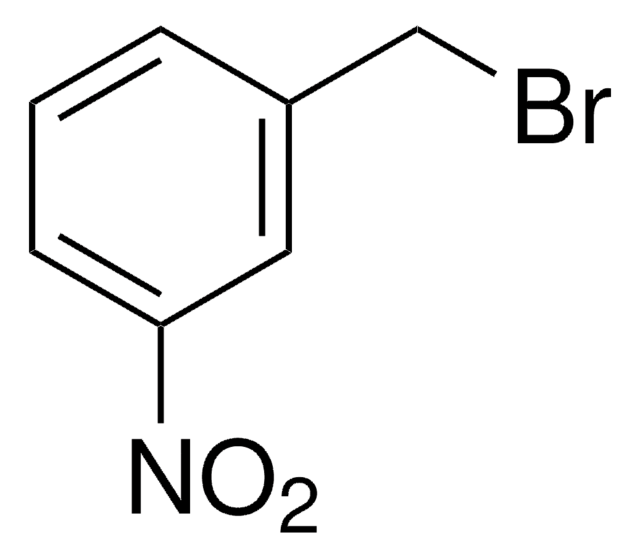
3-Methoxybenzyl bromide
98%
Sinonimo/i:
1-Bromomethyl-3-methoxybenzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3OC6H4CH2Br
Numero CAS:
Peso molecolare:
201.06
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
liquid
Indice di rifrazione
n20/D 1.575 (lit.)
P. ebollizione
152 °C (lit.)
Densità
1.436 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
Stringa SMILE
COc1cccc(CBr)c1
InChI
1S/C8H9BrO/c1-10-8-4-2-3-7(5-8)6-9/h2-5H,6H2,1H3
ZKSOJQDNSNJIQW-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
3-Methoxybenzyl bromide is a benzyl bromide derivative.
Applicazioni
3-Methoxybenzyl bromide (1-bromomethyl-3-methoxybenzene) may be used in the diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 6-(3-methoxyphenyl)-hexane-2,4-dione
- N-(3-methoxybenzyl)-N-(1-methyl-1-phenylethyl)-amine
- 2-(3-methoxybenzyl)-3-[(1R)-7,7-dimethyl-2-oxobicyclo[2.2.1]hept-1-yl]-(3S)-2-thionia-bicyclo [2.2.1]- heptane tetrafluoroborate
- 1-(3-methoxybenzyl)-5-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazole
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yang Zhang et al.
The Journal of organic chemistry, 71(12), 4516-4520 (2006-06-06)
A mild protocol for the conversion of beta-ketoesters and beta-diketones to carboxylic acids with use of CAN in CH3CN is described. The method is compatible with a number of functional groups, and can generate carboxylic acids under neutral conditions at
Highly diastereoselective alkylation of vicinal dianions of chiral succinic acid derivatives: a new general strategy to (R)-?-arylmethyl-?-butyrolactones.
Pohmakotr M, et al.
Tetrahedron Letters, 45(22), 4315-4318 (2004)
Synthesis of (-)-kainic acid using chiral lithium amides in an asymmetric dearomatizing cyclization.
Clayden J, et al.
Tetrahedron, 58(23), 4727-4733 (2002)
Application of sulfur ylide mediated epoxidations in the asymmetric synthesis of ?-hydroxy-d-lactones. Synthesis of a mevinic acid analogue and (+)-prelactone B.
Aggarwal VK, et al.
Tetrahedron Asymmetry, 60(43), 9725-9733 (2004)
Johan R Johansson et al.
The Journal of organic chemistry, 76(7), 2355-2359 (2011-03-11)
An experimentally simple sequential one-pot RuAAC reaction, affording 1,5-disubstituted 1H-1,2,3-triazoles in good to excellent yields starting from an alkyl halide, sodium azide, and an alkyne, is reported. The organic azide is formed in situ by treating the primary alkyl halide
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.