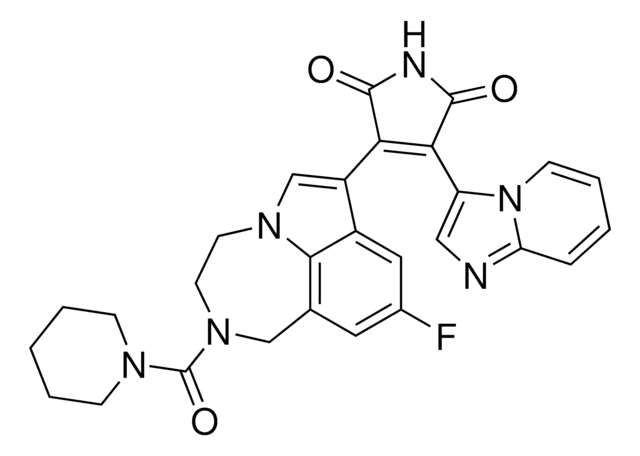
T8325
TDZD-8
≥98% (HPLC), needles
Sinônimo(s):
4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H10N2O2S
Número CAS:
Peso molecular:
222.26
Número MDL:
Código UNSPSC:
12352200
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Ensaio
≥98% (HPLC)
Formulário
needles
condição de armazenamento
protect from light
cor
white
pf
63-64.4 °C
solubilidade
DMSO: >10 mg/mL
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CN1SC(=O)N(Cc2ccccc2)C1=O
InChI
1S/C10H10N2O2S/c1-11-9(13)12(10(14)15-11)7-8-5-3-2-4-6-8/h2-6H,7H2,1H3
chave InChI
JDSJDASOXWCHPN-UHFFFAOYSA-N
Aplicação
TDZD-8 has been used to study the role of GSK-3 in maintaining MLL leukemia stem cell transcriptional program. TDZD-8 has also been used to analyze its effect on neural functions of the mouse olfactory bulb.
Ações bioquímicas/fisiológicas
TDZD-8 is a selective inhibitor of GSK-3, a thiadiazolidinone derivative, non-ATP competitive inhibitor of GSK-3β (IC50 = 2 μM). It does not inhibit Cdk-1/cyclin B, CK-II, PKA or PKC at >100 μM. TDZD-8 has been proposed to bind to the kinase site of GSK-3β.
TDZD-8 is a selective inhibitor of GSK-3.
Características e benefícios
This compound is featured on the GSK-3 page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Nota de preparo
TDZD-8 is soluble in DMSO at a concentration that is greater than 10 mg/ml.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Yujia Wang et al.
Perfusion, 33(8), 679-686 (2018-07-11)
Sevoflurane has been shown to protect against myocardial ischemia/reperfusion (I/R) injury in animals, while its cardioprotection is lost if the ischemic insult is too long. In this study, we proposed a prevailing hypothesis that GSK-3β inhibitor-mediated activation of GSK-3β/β-catenin signaling
Weituan Xu et al.
Neuroscience letters, 634, 52-59 (2016-10-26)
Neuroinflammation is identified to be crucial in the development of neuropathic pain, whereas definite molecular mechanisms remain obscure. Recently, chemokine CXCL5 is manifested to participate in the inflammatory process of central nervous system, however, little is known about the potential
Teng Jiang et al.
Inflammation, 41(3), 811-823 (2018-01-25)
As a recently identified susceptibility gene for Alzheimer's disease (AD), triggering receptor expressed on myeloid cells 2 (TREM2) encodes an immune receptor that is uniquely expressed on microglia, functioning as a modulator of microglial functions including phagocytosis and inflammatory response.
Zhibin Liang et al.
ACS chemical neuroscience, 9(5), 1166-1183 (2018-01-31)
Glycogen synthase kinase-3β (GSK-3β) is a key enzyme responsible for tau hyperphosphorylation and is a viable therapeutic target of Alzheimer's disease (AD). We developed a new class of GSK-3β inhibitors based on the 6- C-glycosylflavone isoorientin (1). The new inhibitors
Jun Yoshida et al.
Lipids, 52(3), 295-301 (2017-02-15)
Many uncommon non-methylene-interrupted fatty acids (NMI FA) are present in limpet gonads, but their biological properties remain unknown. To investigate new biological effects of naturally occurring NMI FA in eukaryotic cells, the biological activities of structurally analogous (4Z,15Z)-octadecadienoic acid (1)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica