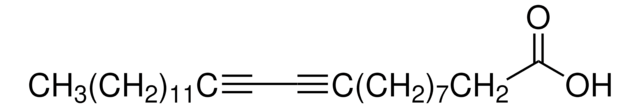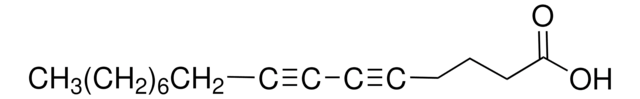O8382
17-Octadecynoic acid
≥95% (GC)
Sinônimo(s):
17-ODYA, Alkynyl Stearic Acid, Octadec-17-ynoic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C18H32O2
Número CAS:
Peso molecular:
280.45
Número MDL:
Código UNSPSC:
12352106
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Ensaio
≥95% (GC)
Formulário
powder
solubilidade
chloroform: 10 mg/mL to clear, colorless to faintly yellow
cadeia de caracteres SMILES
C#CCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h1H,3-17H2,(H,19,20)
chave InChI
DZIILFGADWDKMF-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
17-Octadecynoic acid has been used in lipid synthesis.
Ações bioquímicas/fisiológicas
17-Octadecynoic acid (7-ODYA) is an irreversible inhibitor of cytochrome P450 isozymes, that participates in long-chain fatty acid metabolism.
Suicide substrate inhibitor that selectively and irreversibly inhibits cytochrome P450 epoxygenases and ω-hydrolases.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Effects of intrarenal infusion of 17-octadecynoic acid on renal antihypertensive mechanisms in anesthetized rabbits
Evans R G, et al.
American Journal of Hypertension, 11(7), 803-812 (1998)
M H Wang et al.
The Journal of pharmacology and experimental therapeutics, 284(3), 966-973 (1998-03-13)
We characterized the inhibitory activity of several acetylenic and olefinic compounds on cytochrome P450 (CYP)-derived arachidonic acid omega-hydroxylation and epoxidation using rat renal cortical microsomes and recombinant CYP proteins. Among the acetylenic compounds, 6-(2-propargyloxyphenyl)hexanoic acid (PPOH) and N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide were found
Kristina Hofmann et al.
Scientific reports, 7(1), 10779-10779 (2017-09-09)
The grey and white matter regions of the mammalian brain consist of both neurons and neuroglial cells. Among the neuroglia, the two macroglia oligodendrocytes and astrocytes are the most abundant cell types. While the major function of oligodendrocytes is the
Lu Wei et al.
Nature methods, 11(4), 410-412 (2014-03-04)
Sensitive and specific visualization of small biomolecules in living systems is highly challenging. We report stimulated Raman-scattering imaging of alkyne tags as a general strategy for studying a broad spectrum of small biomolecules in live cells and animals. We demonstrate
Mamta Fuloria et al.
American journal of physiology. Lung cellular and molecular physiology, 283(2), L383-L389 (2002-07-13)
We examined the responses of newborn piglet pulmonary resistance arteries (PRAs) to 5,6-epoxyeicosatrienoic acid (5,6-EET), a cytochrome P-450 metabolite of arachidonic acid. In PRAs preconstricted with a thromboxane A(2) mimetic, 5,6-EET caused a concentration-dependent dilation. This dilation was partially inhibited
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)