N2286
Nonactin
from Streptomyces griseus, ≥98.0% (Total homologs, HPLC)
Sinônimo(s):
Ammonium ionophore I, Ammonium ionophore
About This Item
Produtos recomendados
fonte biológica
Streptomyces griseus
Nível de qualidade
descrição
Natural macrotetrolide, may contain homologues (like monactin and dinactin)
Ensaio
≥98.0% (Total homologs, HPLC)
Formulário
powder
solubilidade
chloroform: soluble 10 mg/mL
espectro de atividade do antibiótico
Gram-positive bacteria
Modo de ação
DNA synthesis | interferes
cell membrane | interferes
temperatura de armazenamento
2-8°C
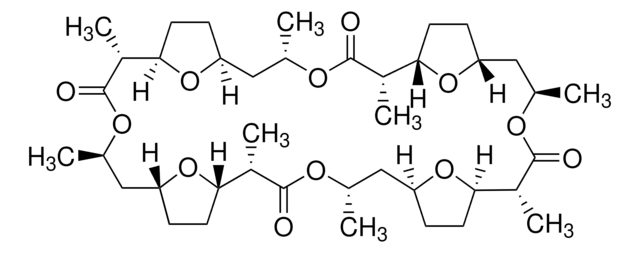
cadeia de caracteres SMILES
C[C@H]1C[C@@H]2CC[C@@H](O2)[C@@H](C)C(=O)O[C@H](C)C[C@H]3CC[C@H](O3)[C@H](C)C(=O)O[C@@H](C)C[C@@H]4CC[C@@H](O4)[C@@H](C)C(=O)O[C@H](C)C[C@H]5CC[C@H](O5)[C@H](C)C(=O)O1
InChI
1S/C40H64O12/c1-21-17-29-9-13-34(49-29)26(6)38(42)46-23(3)19-31-11-15-36(51-31)28(8)40(44)48-24(4)20-32-12-16-35(52-32)27(7)39(43)47-22(2)18-30-10-14-33(50-30)25(5)37(41)45-21/h21-36H,9-20H2,1-8H3/t21-,22+,23+,24-,25-,26+,27+,28-,29-,30+,31+,32-,33-,34+,35+,36-
chave InChI
RMIXHJPMNBXMBU-QIIXEHPYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Outras notas
Aplicação
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica