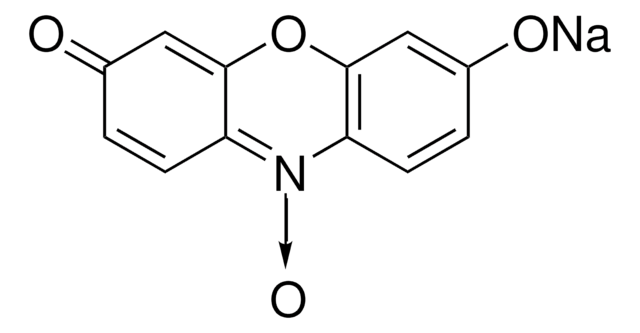
M1544
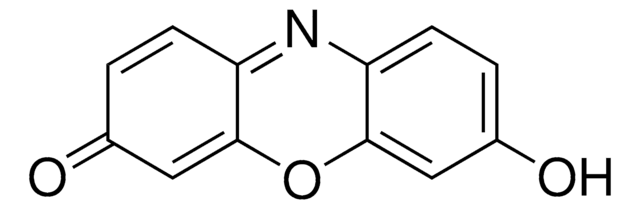
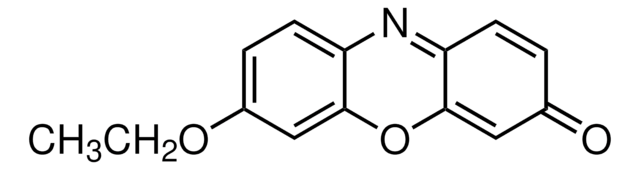
Resorufin methyl ether
Sinônimo(s):
7-Methoxy-3H-phenoxazin-3-one, Methoxyresorufin, O7-Methylresorufin
About This Item
Produtos recomendados
Ensaio
≥98.0% (HPLC)
Nível de qualidade
forma
powder
pf
≥220 °C (lit.)
solubilidade
dichloromethane: 0.95-1.05 mg/mL, clear, orange to very deep orange
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
COc1ccc2N=C3C=CC(=O)C=C3Oc2c1
InChI
1S/C13H9NO3/c1-16-9-3-5-11-13(7-9)17-12-6-8(15)2-4-10(12)14-11/h2-7H,1H3
chave InChI
KNYYMGDYROYBRE-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Phase I biotransformation reactions introduce or expose functional groups on the drug with the goal of increasing the polarity of the compound. Although Phase I drug metabolism occurs in most tissues, the primary and first pass site of metabolism occurs during hepatic circulation.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica