L9283
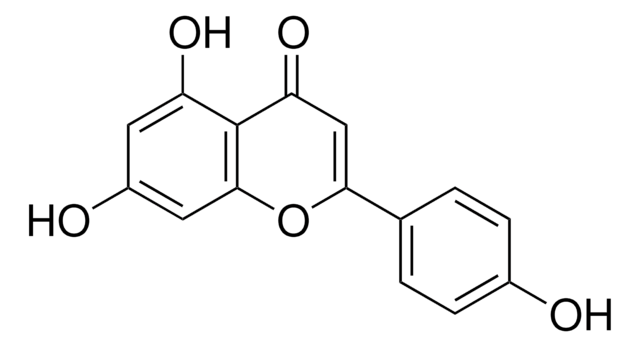
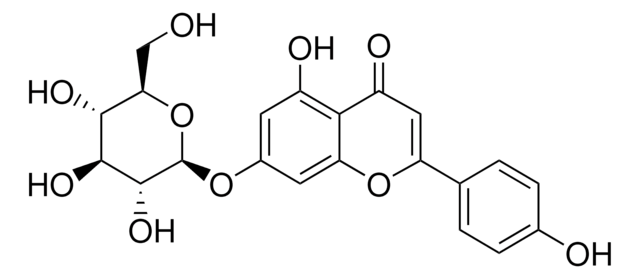
Luteolin
≥98% (TLC), powder, antioxidant
Sinônimo(s):
3′,4′,5,7-Tetrahydroxyflavone
About This Item
Produtos recomendados
product name
Luteolin, ≥98% (TLC), powder
Nível de qualidade
Ensaio
≥98% (TLC)
forma
powder
prazo de validade
3 yr
cor
yellow
pf
~330 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Oc1cc(O)c2C(=O)C=C(Oc2c1)c3ccc(O)c(O)c3
InChI
1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
chave InChI
IQPNAANSBPBGFQ-UHFFFAOYSA-N
Informações sobre genes
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , GSK3A(2931)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to induce and elucidate the apoptotic pathway in renal cell carcinoma 786-O cells
- as an additive in M9 minimal medium to induce nodF gene expression
- as a reference standard to qualitatively and quantitatively analyse luteolin using reverse phase-high performance liquid chromatography with diode array detector (RP-HPLC-DAD)
- as a reaction supplement for β-galactosidase assay
- to elucidate the anti-inflammatory efficacy of luteolin in pseudorabies virus infected RAW264.7 cell line by measuring the anti-inflammatory mediators production and also cell viability and cytotoxicity assay
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Conteúdo relacionado
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica