K0133
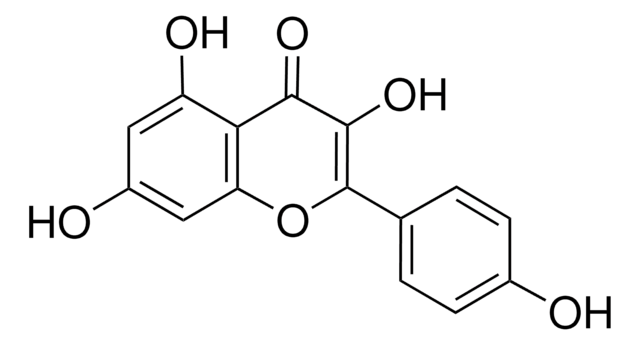
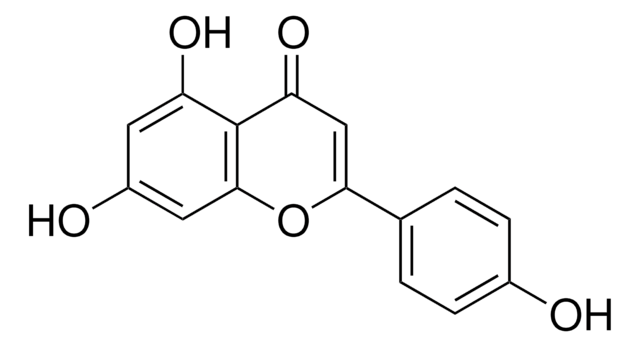
Kaempferol
≥90% (HPLC), powder
Sinônimo(s):
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
Produtos recomendados
fonte biológica
synthetic
Nível de qualidade
Ensaio
≥90% (HPLC)
forma
powder
condição de armazenamento
protect from light
cor
yellow
pf
277 °C
solubilidade
ethanol: 20 mg/mL
DMSO: 50 mg/mL
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
chave InChI
IYRMWMYZSQPJKC-UHFFFAOYSA-N
Informações sobre genes
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to check its potential effect as an antioxidant and neuroprotective agent against rotenone-induced Parkinson′s disease (PD) model in SH-S5Y5 cells
- to test its anti-inflammatory effect on lipopolysaccharide (LPS)-induced inflammatory injury in human aortic endothelial cells (HAECs)
- to study its apoptosis sensitizing effect on non-small cell lung cancer (NSCLC) cells by inhibiting nuclear factor erythroid 2-related factor 2 (Nrf2)
Ações bioquímicas/fisiológicas
Embalagem
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica