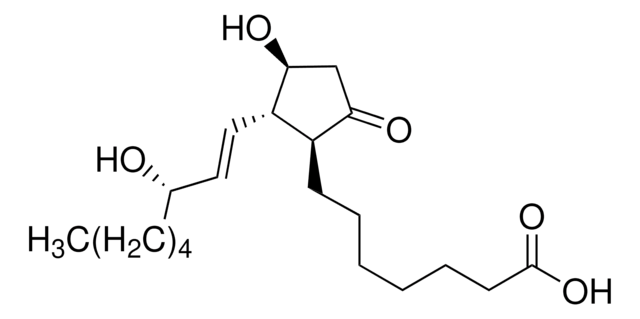
D8174
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α
≥98% (HPLC), solution, thromboxane A2 agonist
Sinônimo(s):
U-46619, U46619
About This Item
Produtos recomendados
Nome do produto
9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α, solution, 10 mg/mL in methyl acetate
Formulário
solution
Nível de qualidade
concentração
10 mg/mL in methyl acetate
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
CCCCC[C@H](O)\C=C\[C@H]1C2CC(CO2)[C@@H]1C\C=C/CCCC(O)=O
InChI
1S/C21H34O4/c1-2-3-6-9-17(22)12-13-19-18(16-14-20(19)25-15-16)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16?,17-,18-,19+,20?/m0/s1
chave InChI
LQANGKSBLPMBTJ-REGKDVDGSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to induce aortic smooth muscle (SM) contraction in mice deficient in myosin light chain 9 (Myl9) gene
- to induce contraction as part of vascular reactivity experiments using mice aorta
- as a thromboxane/prostaglandin agonist to study the effect of dithiothreitol (DTT) on mice arterial vessel viability
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Órgãos-alvo
Central nervous system
Perigos de suplementos
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
14.0 °F
Ponto de fulgor (°C)
-10 °C
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Conteúdo relacionado
Discover Bioactive Small Molecules for Lipid Signaling Research
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica