D1692
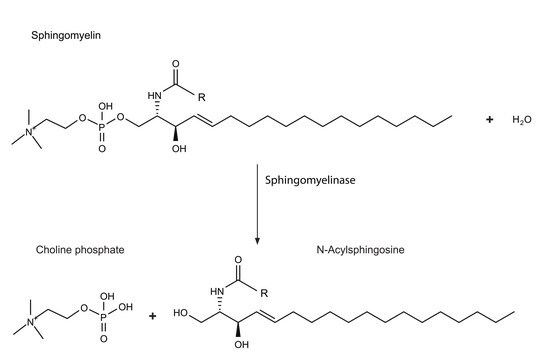
GW4869
≥90% (NMR), powder, N-SMase inhibitor
Sinônimo(s):
N,N′-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-3,3′-p-phenylene-bis-acrylamide dihydrochloride
About This Item
Produtos recomendados
product name
GW4869, ≥90% (NMR)
Nível de qualidade
Ensaio
≥90% (NMR)
forma
powder
condição de armazenamento
desiccated
protect from light
cor
light yellow to yellow
pf
>300 °C
solubilidade
DMSO: 0.2 mg/mL
originador
GlaxoSmithKline
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cl.Cl.O=C(Nc1ccc(cc1)C2=NCCN2)\C=C/c3ccc(\C=C/C(=O)Nc4ccc(cc4)C5=NCCN5)cc3
InChI
1S/C30H28N6O2.2ClH/c37-27(35-25-11-7-23(8-12-25)29-31-17-18-32-29)15-5-21-1-2-22(4-3-21)6-16-28(38)36-26-13-9-24(10-14-26)30-33-19-20-34-30;;/h1-16H,17-20H2,(H,31,32)(H,33,34)(H,35,37)(H,36,38);2*1H/b15-5-,16-6-;;
chave InChI
NSFKAZDTKIKLKT-LOLTXFFGSA-N
Descrição geral
Aplicação
- as an inhibitor of neutral sphingomyelinase and exosome biogenesis
- to analyse the effects of arsenic trioxide (ATO) treatment for hepatoma carcinoma HCCLM3 cells on ceramide production
- to determine the contributions of p75 neurotrophin receptor (p75NTR) and tropomyosin receptor kinase A (TrkA)- coupled pathways to nerve growth factor (NGF)-induced thermal hypersensitivity in rats
Ações bioquímicas/fisiológicas
Características e benefícios
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Conteúdo relacionado
Discover Bioactive Small Molecules for Lipid Signaling Research
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica