C2196
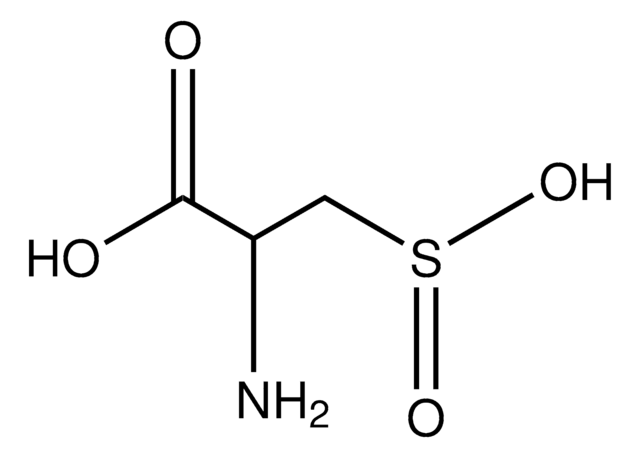
L-Cysteine S-sulfate
≥98% (TLC), suitable for ligand binding assays
Sinônimo(s):
S-Sulfo-L-cysteine
About This Item
Produtos recomendados
Nome do produto
L-Cysteine S-sulfate, ≥98% (TLC)
Nível de qualidade
Ensaio
≥98% (TLC)
Formulário
powder
técnica(s)
ligand binding assay: suitable
cor
white
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
N[C@@H](CSS(O)(=O)=O)C(O)=O
InChI
1S/C3H7NO5S2/c4-2(3(5)6)1-10-11(7,8)9/h2H,1,4H2,(H,5,6)(H,7,8,9)/t2-/m0/s1
chave InChI
NOKPBJYHPHHWAN-REOHCLBHSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Ações bioquímicas/fisiológicas
Cysteine is one of the functional amino acids that are associated with growth, reproduction, maintenance and immunity. Cysteine is a source of disulfide linkage in protein and is associated with sulfur transport. At physiological pH, cysteine undergoes rapid oxidation to form cystine. Reduced availability of cysteine or cystine, influences leukocyte metabolism. L-Cysteine serves as a precursor for the rate limiting step in glutathione synthesis that occurs in neurons. It donates inorganic sulfate for detoxification reactions. Therefore, L-cysteine might be associated with neuroprotection. It is found to obstruct the entry of heavy metal ions across the blood-brain barrier into the brain. Increased levels of L-cysteine might lead to neurotoxicity.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica