70050
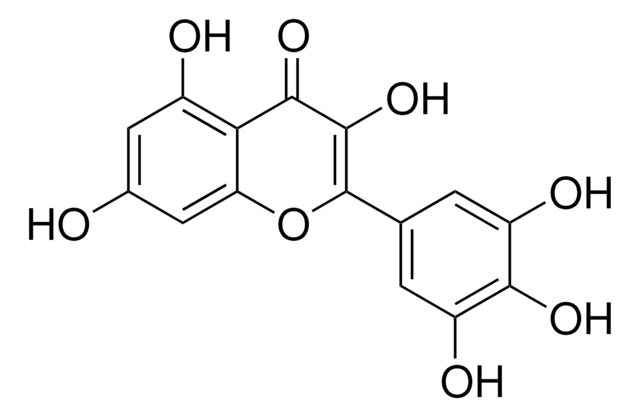
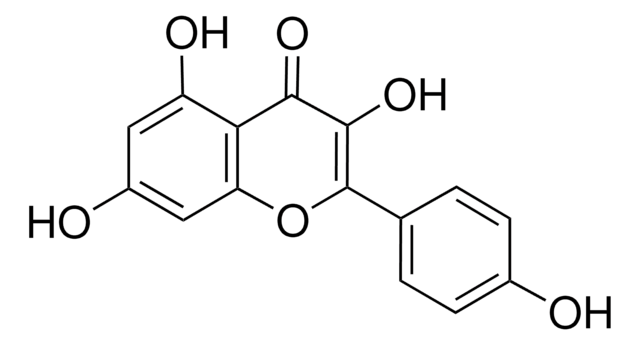
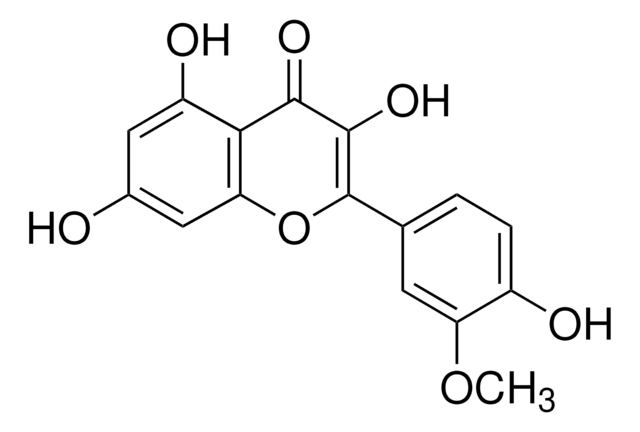
Myricetin
≥96.0% (HPLC)
Sinônimo(s):
3,3′,4′,5,5′,7-Hexahydroxyflavone, Cannabiscetin, Myricetol
About This Item
Produtos recomendados
Ensaio
≥96.0% (HPLC)
forma
powder
pf
≥300 °C
>300 °C (lit.)
solubilidade
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
aplicação(ões)
metabolomics
vitamins, nutraceuticals, and natural products
cadeia de caracteres SMILES
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
chave InChI
IKMDFBPHZNJCSN-UHFFFAOYSA-N
Informações sobre genes
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocolos
Coumaric acid; Quercitrin; Myricetin; Quercetin
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica