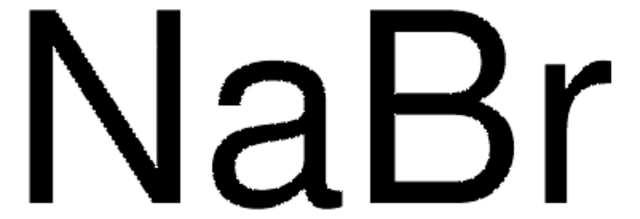P1147
Acetato de potássio
ReagentPlus®, ≥99.0%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
CH3COOK
Número CAS:
Peso molecular:
98.14
Beilstein:
3595449
Número CE:
Número MDL:
Código UNSPSC:
12352302
ID de substância PubChem:
NACRES:
NA.21
Produtos recomendados
grau
reagent
pressão de vapor
<0.0000001 hPa ( 25 °C)
linha de produto
ReagentPlus®
Ensaio
≥99.0%
Formulário
powder or crystals
pH
7-9 (25 °C, 98.2 g/L)
densidade
1.57 g/cm3 at 25 °C (lit.)
cadeia de caracteres SMILES
[K+].CC([O-])=O
InChI
1S/C2H4O2.K/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1
chave InChI
SCVFZCLFOSHCOH-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Potassium acetate is an osmotic agent that can be prepared by the reaction between acetic acid and potassium hydroxide. It has replaced urea and glycol for deicing process. Its potential as a substitute to calcium chloride for osmotic distillation has been investigated. It intercalates with the hydroxyl groups on the kaolinite surface leading to surface modification. It acts as a catalyst that accelerates the acetylation of wood at low temperature.
Aplicação
Potassium acetate has been used during granule bound starch synthase (GBSS) activity assay. It may be used as an activator to produce waste tea activated carbon (WTAC).
Informações legais
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Potassium acetate-catalyzed acetylation of wood: reaction rates at low temperatures.
Obataya E and Minato K.
Wood Science and Technology, 43(5-6), 405-413 (2009)
M M Shah et al.
Applied biochemistry and biotechnology, 63-65, 423-433 (1997-04-01)
Potassium acetate is currently made by reacting petroleum-based acetic acid with potassium hydroxide. An alternate process, anaerobic fermentation of dextrose with Clostridium thermoaceticum, could be used and could possibly be cheaper. Growth characteristics and productivity of the fermentation were optimized
Purification of starch granules from Arabidopsis leaves and determination of granule-bound starch synthase activity.
Albi T, et al.
Bio-protocol, 4(23), e1316-e1316 (2013)
Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye.
Auta M and Hameed BH.
Chemical Engineering Journal, 171(2), 502-509 (2011)
Modification of kaolinite surfaces through intercalation with potassium acetate, II.
Frost RL, et al.
Journal of Colloid and Interface Science, 214(1), 109-117 (1999)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica