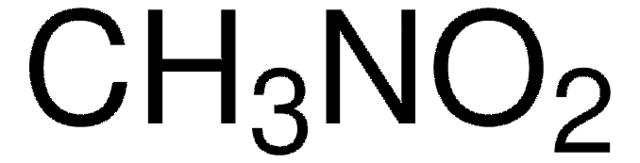About This Item
Produtos recomendados
densidade de vapor
2.1 (vs air)
Nível de qualidade
pressão de vapor
2.7 mmHg
Ensaio
≥96%
Formulário
liquid
temperatura de autoignição
784 °F
Lim. expl.
7.3 %, 33 °F
técnica(s)
HPLC: suitable
Impurezas
<0.030% water
índice de refração
n20/D 1.382 (lit.)
pH
6.4 (20 °C, 0.01 g/L)
p.e.
101.2 °C (lit.)
pf
−29 °C (lit.)
densidade
1.127 g/mL at 25 °C (lit.)
λ
H2O reference
Absorção UV
λ: 380 nm Amax: 1.00
λ: 386 nm Amax: 0.50
λ: 395 nm Amax: 0.20
λ: 400 nm Amax: 0.10
λ: 405 nm Amax: 0.05
λ: 430-700 nm Amax: 0.01
aplicação(ões)
food and beverages
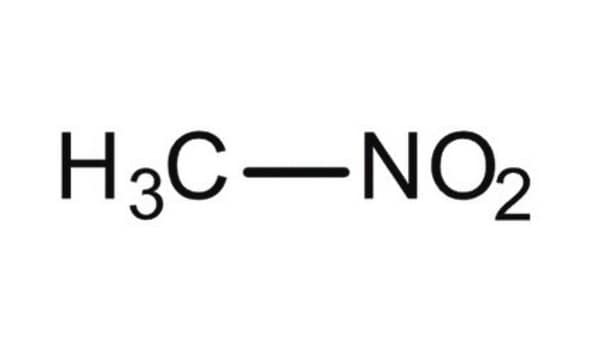
cadeia de caracteres SMILES
C[N+]([O-])=O
InChI
1S/CH3NO2/c1-2(3)4/h1H3
chave InChI
LYGJENNIWJXYER-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Asymmetric aza-Henry reaction toward trifluoromethyl β-nitroamines and biological investigation of their adamantane-type derivatives.: This study used nitromethane in asymmetric aza-Henry reactions to synthesize trifluoromethyl β-nitroamines, which were further investigated for their biological properties, showcasing the potential of nitromethane in advanced synthetic chemistry (Ren et al., 2024).
- Effect of Temperature on the Liquid Bridging Force while Maintaining Physical Stability in Solid-Liquid Mixed Fuel.: Nitromethane was analyzed in this research to understand its role in the stability of solid-liquid mixed fuels under varying temperatures, providing insights into the optimization of fuel formulations (Zhang et al., 2024).
- Generation of New Synthons for Synthesis Through Activation of Nitromethane.: This research demonstrated the activation of nitromethane to generate new synthons for synthetic applications, highlighting its versatility and importance in creating novel chemical entities (Wang et al., 2024).
- Towards Chemoenzymatic Syntheses of Both Enantiomers of Phosphoemeriamine.: The study explored the use of nitromethane in chemoenzymatic syntheses, enabling the production of both enantiomers of phosphoemeriamine, an important compound in chemical biology (Kiełbasiński et al., 2024).
- Rationally introducing non-canonical amino acids to enhance catalytic activity of LmrR for Henry reaction.: Nitromethane was employed in this study to investigate the enhancement of catalytic activity in the Henry reaction through the introduction of non-canonical amino acids, demonstrating its significance in enzyme catalysis research (Wang et al., 2024).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Código de classe de armazenamento
4.1A - Other explosive hazardous materials
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
95.0 °F - closed cup
Ponto de fulgor (°C)
35 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Protocolos
GC Analysis of Class 2 Residual Solvents on OVI-G43
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica