About This Item
Fórmula linear:
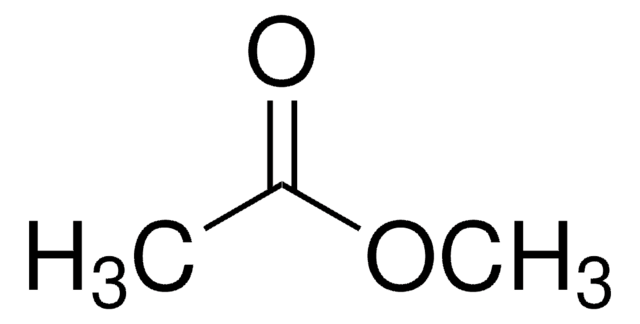
CH3COOCH3
Número CAS:
Peso molecular:
74.08
Beilstein:
1736662
Número CE:
Número MDL:
Código UNSPSC:
12352108
ID de substância PubChem:
NACRES:
NA.21
Produtos recomendados
grau
reagent
Nível de qualidade
densidade de vapor
2.55 (vs air)
pressão de vapor
165 mmHg ( 20 °C)
linha de produto
ReagentPlus®
Ensaio
99%
Formulário
liquid
temperatura de autoignição
936 °F
Lim. expl.
16 %
dilution
(for general lab use)
índice de refração
n20/D 1.361 (lit.)
p.e.
57-58 °C (lit.)
pf
−98 °C (lit.)
densidade
0.934 g/mL at 25 °C
cadeia de caracteres SMILES
COC(C)=O
InChI
1S/C3H6O2/c1-3(4)5-2/h1-2H3
chave InChI
KXKVLQRXCPHEJC-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Its IR spectra in the vapor phase and in solution form (in CS2 and CCl4) have been reported. It can be synthesized from dimethyl ether via carbonylation in the presence of halide-free catalysts based on zeolites. It has also been reported to be formed during the synthesis of poly(vinyl) alcohol (PVA). It undergoes transesterification reaction with n-octanol in the presence of Amberlyst 15 catalyst to afford octyl acetate and methanol.
Methyl acetate is an aliphatic ester that can be prepared via carbonylation of dimethyl ether over zeolites. MA is formed as a by-product during the preparation of polyvinyl alcohol from acetic acid and methanol.
Aplicação
Methyl acetate may be used for the preparation of fatty acid methyl esters and triacetin from rapeseed oil via non-catalytic trans-esterification reaction under super-critical conditions.
Methyl acetate may be used in the following:
- As acyl acceptor in the preparation of biodiesel.
- Synthesis of ethanol.
- Preparation of n-butyl acetate, via transesterification reaction with n-butanol in the presence of acidic catalysts.
- acetic anhydride
- methyl acrylate
- vinyl acetate
- ethyl amide
Informações legais
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Órgãos-alvo
Central nervous system
Perigos de suplementos
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
8.6 °F - closed cup
Ponto de fulgor (°C)
-13 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Kinetics of transesterification of methyl acetate and n-octanol catalyzed by cation exchange resins.
Liu Y, et al.
Korean Journal of Chemical Engineering, 30(5), 1039-1042 (2013)
Catalysts, Kinetics, and Reactive Distillation for Methyl Acetate Synthesis.
Zuo C, et al.
Industrial & Engineering Chemistry Research, 53(26), 10540-10548 (2014)
A new process for catalyst-free production of biodiesel using supercritical methyl acetate.
Saka S and Isayama Y.
Fuel: The Science and Technology of Fuel and Energy, 88(7), 1307-1313 (2009)
Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites.
Patricia Cheung et al.
Angewandte Chemie (International ed. in English), 45(10), 1617-1620 (2006-01-31)
Synthesis of ethanol from methanol and syngas through an indirect route containing methanol dehydrogenation, DME carbonylation, and methyl acetate hydrogenolysis.
Liu Y, et al.
Fuel Processing Technology, 110, 206-213 (2013)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica