567300
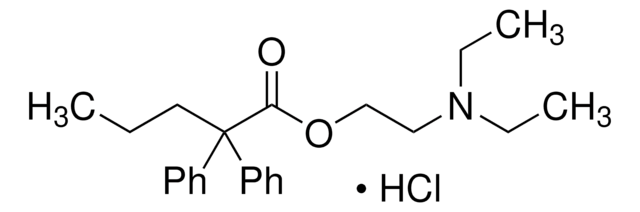
SKF-525A, Hydrochloride
Cell permeable. Blocks glibenclamide-sensitive K+ channels. Inhibits neuronal nitric oxide synthase. Also inhibits hepatic drug metabolism by inhibiting the cytochrome P450 system.
Sinônimo(s):
SKF-525A, Hydrochloride, Proadifen
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98% (TLC)
forma
solid
fabricante/nome comercial
Calbiochem®
condição de armazenamento
OK to freeze
desiccated (hygroscopic)
cor
white
solubilidade
water: 20 mg/mL
Condições de expedição
ambient
temperatura de armazenamento
−20°C
InChI
1S/C23H31NO2.ClH/c1-4-17-23(20-13-9-7-10-14-20,21-15-11-8-12-16-21)22(25)26-19-18-24(5-2)6-3;/h7-16H,4-6,17-19H2,1-3H3;1H
chave InChI
FHIKZROVIDCMJA-UHFFFAOYSA-N
Descrição geral
Ações bioquímicas/fisiológicas
nNOS
Advertência
Reconstituição
Outras notas
Sakuta, H. and Yoneda, I. 1994. Eur. J. Pharmacol.252, 117.
Khan, S., et al. 1993. Biochem. Pharmacol.45, 439.
Boeynaems, J.M., et al. 1986. Prostaglandins32, 145.
Lee, C.H., et al. 1976. J. Pharmacol. Exp. Ther.198, 347.
Grasdalen, J., et al. 1975. FEBS Lett.60, 240.
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica