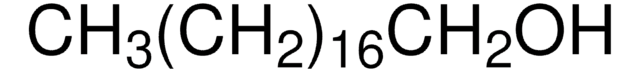1.46481
Buffered NaCl-Peptone Solution with TLH
ready-to-use, bottle volume 1000 mL , filling volume
Sinônimo(s):
Buffered Saline Peptone Water with neutralizer, Buffered Sodium Chloride Peptone Solution with 0.3% Lecithin, 3% Tween® and 0.1% Histidin
About This Item
Produtos recomendados
Agency
EP 2.6.13
Nível de qualidade
descrição
For the microbiological examination of non-sterile pharmaceuticals
esterilidade
sterile; autoclaved
Formulário
liquid
prazo de validade
12 mo.
Características
closure type screw cap with septum
ready-to-use
embalagem
bottle of
pkg of (1 L in 1 L bottle with blue screw cap and 3 loci (6 bottles per box))
técnica(s)
direct inoculation: suitable
sterile filtration: suitable
capacidade do frasco
1000 mL
volume do frasco
1000 mL , filling volume
pH
7.0
aplicação(ões)
bioburden testing
cosmetics
food and beverages
pharmaceutical
componentes
lecithin
histidine
tween
temperatura de armazenamento
2-25°C
adequação
nonselective for
Descrição geral
Aplicação
Características e benefícios
Informações legais
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica