W288608
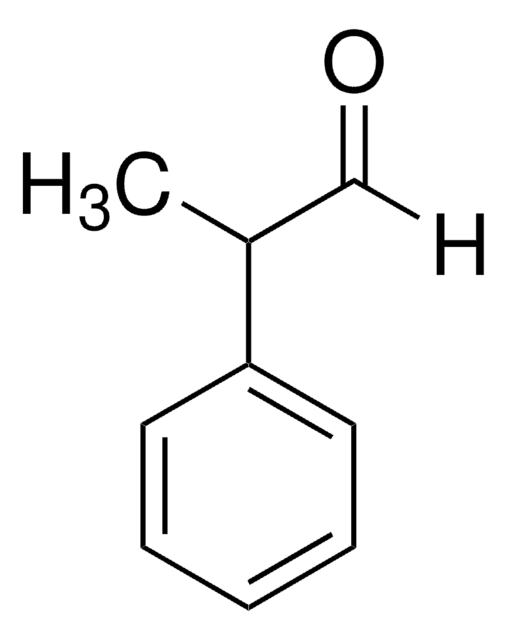
2-Phenylpropionaldehyde
≥95%, FCC, FG
Sinônimo(s):
2-Phenylpropanal, Hydratropaldehyde
About This Item
Produtos recomendados
fonte biológica
synthetic
Nível de qualidade
grau
FG
Halal
Kosher
Agency
meets purity specifications of JECFA
conformidade reg.
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
Ensaio
≥95%
índice de refração
n20/D 1.517 (lit.)
p.e.
92-94 °C/12 mmHg (lit.)
densidade
1.002 g/mL at 25 °C (lit.)
aplicação(ões)
flavors and fragrances
Documentação
see Safety & Documentation for available documents
alérgeno alimentar
no known allergens
Organoléptico
fresh; green; floral
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[H]C(=O)C(C)c1ccccc1
InChI
1S/C9H10O/c1-8(7-10)9-5-3-2-4-6-9/h2-8H,1H3
chave InChI
IQVAERDLDAZARL-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- Cytotoxicity, early safety screening, and antimicrobial potential of minor oxime constituents of essential oils and aromatic extracts.: Explores the safety and effectiveness of 2-Phenylpropionaldehyde among other compounds in essential oils, highlighting its potential antimicrobial properties and implications for food safety and preservation (Strub DJ et al., 2022).
- Spectroscopic Evidence for a Cobalt-Bound Peroxyhemiacetal Intermediate.: This study provides spectroscopic evidence of a cobalt-bound intermediate in reactions involving 2-Phenylpropionaldehyde, advancing our knowledge of chemical reaction mechanisms and catalysis (Cho J et al., 2021).
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
174.2 °F
Ponto de fulgor (°C)
79 °C
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica