123129
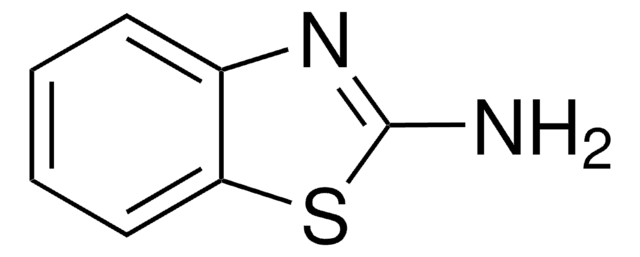
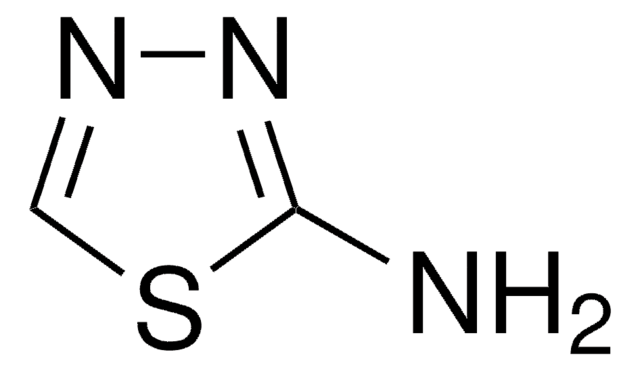
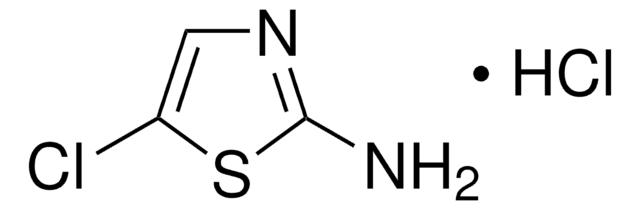
2-Aminothiazole
97%
Sinônimo(s):
2-Thiazolamine
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C3H4N2S
Número CAS:
Peso molecular:
100.14
Beilstein:
105738
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
pf
91-93 °C (lit.)
solubilidade
1 M HCl: soluble 50 mg/mL, clear (dark yellow-brown)
cadeia de caracteres SMILES
Nc1nccs1
InChI
1S/C3H4N2S/c4-3-5-1-2-6-3/h1-2H,(H2,4,5)
chave InChI
RAIPHJJURHTUIC-UHFFFAOYSA-N
Aplicação
2-Aminothiazole was used in the synthesis of 2-aminothiazole-modified silica gel. It was used in Ulmann coupling with 2-chlorobenzoic acids mediated by ultrasonic irradiation.
Ações bioquímicas/fisiológicas
2-Aminothiazoles are potent cyclin-dependent kinase 5 inhibitors and are therapeutic agents for the treatment of Alzheimer′s disease and other neurodegenerative disorders.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Xin Cao et al.
Bioorganic & medicinal chemistry, 16(11), 5890-5898 (2008-05-20)
Because both c-Src and iNOS are key regulatory enzymes in tumorigenesis, a new series of 4-heteroarylamino-3-quinolinecarbonitriles as potent dual inhibitors of both enzymes were designed, prepared, and evaluated for blocking multiple signaling pathways in cancer therapy. All compounds were evaluated
Synthetic Communications, 37, 1853-1853 (2007)
Christopher J Helal et al.
Bioorganic & medicinal chemistry letters, 14(22), 5521-5525 (2004-10-16)
High-throughput screening with cyclin-dependent kinase 5 (cdk5)/p25 led to the discovery of N-(5-isopropyl-thiazol-2-yl)isobutyramide (1). This compound is an equipotent inhibitor of cdk5 and cyclin-dependent kinase 2 (cdk2)/cyclin E (IC(50)=ca. 320nM). Parallel and directed synthesis techniques were utilized to explore the
P S Roldan et al.
Analytical and bioanalytical chemistry, 375(4), 574-577 (2003-03-01)
This work describes the synthesis and characterization of 2-aminothiazole-modified silica gel (SiAT), as well as its application for preconcentration (in batch and column technique) of Cu(II), Ni(II) and Zn(II) in ethanol medium. The adsorption capacities of SiAT determined for each
Ian Bruce et al.
Bioorganic & medicinal chemistry letters, 22(17), 5445-5450 (2012-08-07)
Using a parallel synthesis approach to target a non-conserved region of the PI3K catalytic domain a pan-PI3K inhibitor 1 was elaborated to provide alpha, delta and gamma isoform selective Class I PI3K inhibitors 21, 24, 26 and 27. The compounds
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica