P38803
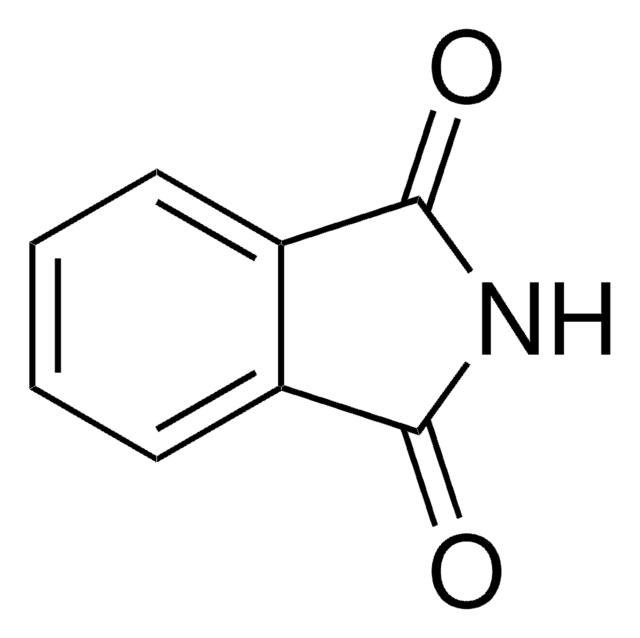
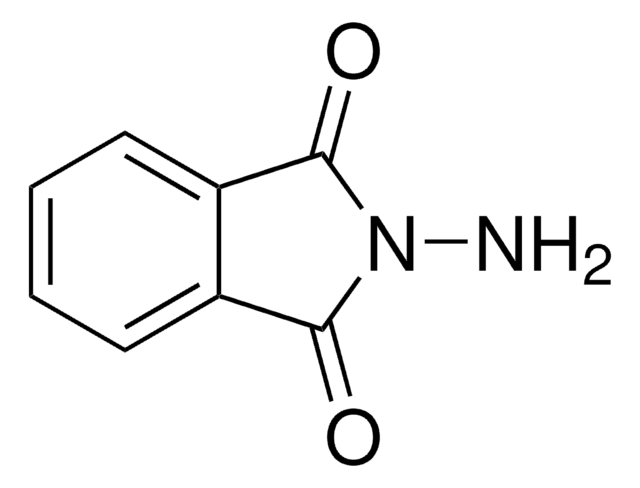
Phthalhydrazide
ReagentPlus®, 99%
Sinônimo(s):
2,3-Dihydro-1,4-phthalazinedione
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C8H6N2O2
Número CAS:
Peso molecular:
162.15
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
linha de produto
ReagentPlus®
Ensaio
99%
Formulário
solid
pf
>300 °C (lit.)
cadeia de caracteres SMILES
O=C1NNC(=O)c2ccccc12
InChI
1S/C8H6N2O2/c11-7-5-3-1-2-4-6(5)8(12)10-9-7/h1-4H,(H,9,11)(H,10,12)
chave InChI
KGLPWQKSKUVKMJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Informações legais
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
I H Hall et al.
Biomedica biochimica acta, 47(4-5), 423-433 (1988-01-01)
2,3-Dihydrophthalazine-1,4-dione effectively lowers serum levels of cholesterol and triglycerides in Sprague Dawley rats after two weeks, after which the cholesterol levels continued to decline. The maximum serum lipid lowering effect on cholesterol or triglyceride levels was during the seventh and
I H Hall et al.
Anti-cancer drugs, 3(1), 55-62 (1992-02-01)
2,3-Dihydrophthalazine-1,4-dione derivatives demonstrated potent cytotoxicity against the growth of murine leukemia cells and human single cell suspension, i.e. Tmolt3 leukemia and HeLa-S3, as well as colon adenocarcinoma and KB nasopharynx. However, only select compounds demonstrated activity against bronchogenic lung, osteosarcoma
J Schiller et al.
Free radical research, 30(1), 45-57 (1999-04-08)
The reactivity of 5-amino-2,3-dihydro-phthalazine-1,4-dione (luminol) and phthalic hydrazide with hydroxyl radicals was studied. HO*-radicals were generated by the Fenton reaction as well as by water radiolysis. Both luminol and phthalic hydrazide react with hydroxyl radicals under intense chemiluminescence (CL) emission.
I H Hall et al.
The Journal of pharmacy and pharmacology, 41(6), 394-397 (1989-06-01)
The disposition of [14C]2,3-dihydrophthalazine-1,4-dione, a potent hypolipidaemic agent, has been determined after both intravenous and oral administration. Both the routes of administration afforded multi-exponential disposition with an estimated t1/2 of approximately 75 h. After oral administration, the drug was observed
L Butner et al.
International journal of tissue reactions, 18(2-3), 47-55 (1996-01-01)
2,3-Dihydrophthalazine-1,4-diones were observed to be potent anti-inflammatory agents as well as capable of protecting against endotoxin shock in mice at 8 mg/kg i.p. These agents blocked both locally- and centrally-induced pain at 8 mg/kg i.p. In part they appear to
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica