P35405
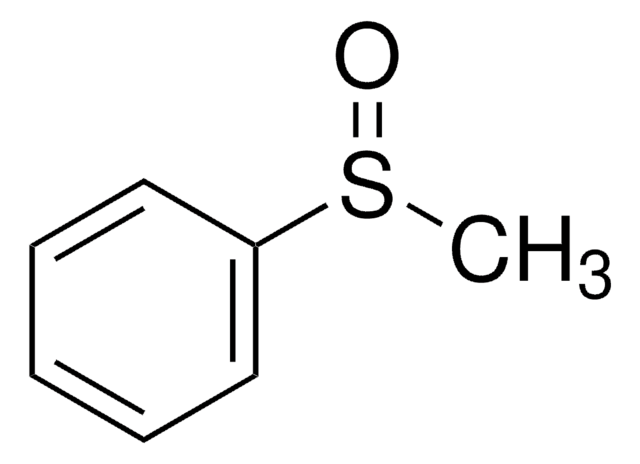
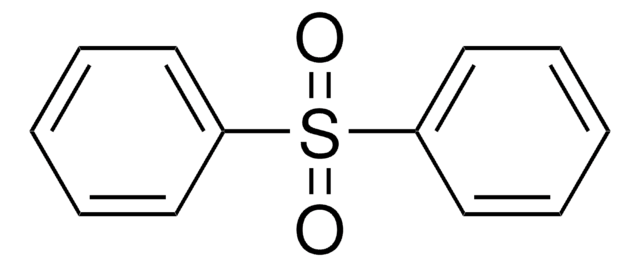
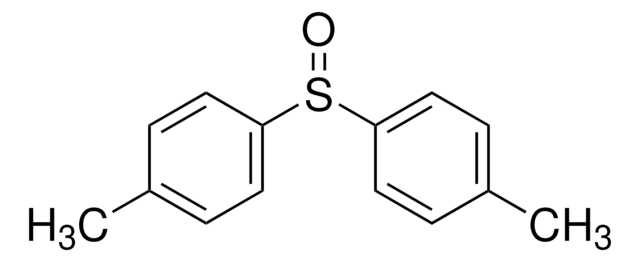
Diphenyl sulfoxide
96%
Sinônimo(s):
Phenyl sulfoxide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
(C6H5)2SO
Número CAS:
Peso molecular:
202.27
Beilstein:
1908444
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
96%
Formulário
crystals
p.e.
206-208 °C/13 mmHg (lit.)
pf
69-71 °C (lit.)
cadeia de caracteres SMILES
O=S(c1ccccc1)c2ccccc2
InChI
1S/C12H10OS/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
chave InChI
JJHHIJFTHRNPIK-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Preparation of radiochemicals: Diphenyl sulfoxide plays a role in the synthesis of [(11)C]cyanide from [(11)C]methyl iodide, facilitating rapid and efficient production of radiochemicals for medical imaging applications (Kikuchi et al., 2022).
- Catalytic oxidation processes: The photocatalytic and catalytic oxidation of diphenyl sulphide to sulfoxide and sulfone was examined, highlighting the effectiveness of hydrogen peroxide and TiO2 polymorphs in optimizing chemical processes (Mikrut et al., 2022).
- Dielectric properties research: The study on dielectric properties of high organic sulfur coal highlighted the modeling of sulfur compounds, which could include diphenyl sulfoxide, enhancing our understanding of materials science in energy sectors (Cai et al., 2019).
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
S Yoshihara et al.
Archives of biochemistry and biophysics, 249(1), 8-14 (1986-08-15)
To characterize the properties of diphenyl sulfoxide (DPSO) as a new type of electron acceptor for guinea pig liver aldehyde oxidase (AO), we compared the kinetics of the reductions of DPSO and other classical electron acceptors such as O2 and
Wen-xian Li et al.
Guang pu xue yu guang pu fen xi = Guang pu, 22(6), 905-907 (2003-08-14)
(Tb1-x Tmx).L2.(ClO4).2H2O(x = 0.000 to 0.200, L = C6H5SOCH2COO-) have been synthesized. The coordination compounds have been studied by means of composition analysis, molar conductivity, IR, and the condition of coordination have been inferred. In the fluorescent spectra it was
J A Kozlowski et al.
Bioorganic & medicinal chemistry letters, 10(20), 2255-2257 (2000-10-31)
Structure activity studies on [4-(phenylsulfonyl)phenyl]methylpiperazine led to the discovery of 4-cyclohexyl-alpha-[4-[[4-methoxyphenyl(S)-sufinyl]phenyl]-1-pi perazineacetonitrile, 1, an M2 selective muscarinic antagonist. Affinity at the cloned human M2 receptor was 2.7 nM; the M1/M2 selectivity is 40-fold.
Wen-Xian Li et al.
Luminescence : the journal of biological and chemical luminescence, 26(6), 754-761 (2011-05-14)
A novel ternary complex, TbL(5) L'(ClO(4))(3) · 3H(2)O, two binary complexes, TbL(7) (ClO(4))(3) · 3H(2)O and TbL'(3.5) (ClO(4))(3) · 4H(2)O has been synthesized (using diphenyl sulphoxide as the first ligand L, bipyridine as the second ligand L'). Their composition was
Tomofumi Takuwa et al.
Chemical & pharmaceutical bulletin, 53(5), 476-480 (2005-05-03)
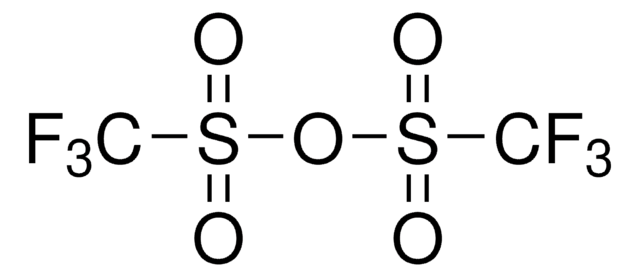
A facile one-pot C-benzylation of various sodium enolates derived from methyl malonate, beta-ketoesters, a beta-cyanoester, a beta-cyanosulfone, ketones and a carboxylic ester is reported. Reaction of alkoxydiphenylsulfonium salts formed by treating various benzyl alcohols with diphenyl sulfide bis(trifluoromethanesulfonate) (derived from
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica