T84603
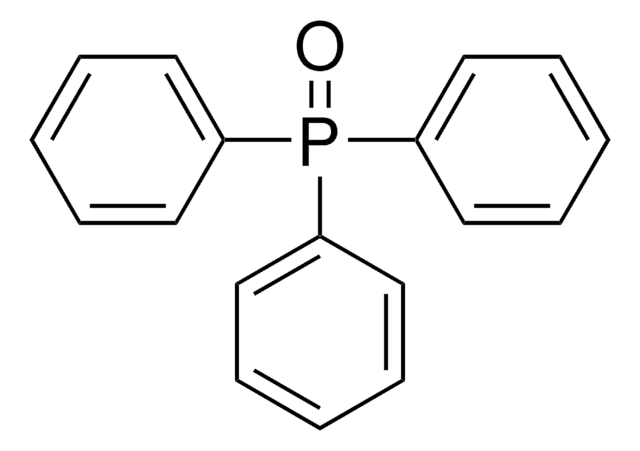
Triphenylphosphine oxide
98%
Sinônimo(s):
Ph3PO, TPPO, Triphenyl phosphorus oxide, Triphenylphosphine monoxide
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
98%
adequação da reação
reagent type: ligand
reaction type: Coupling Reactions
reagent type: ligand
reaction type: Epoxidations
reagent type: ligand
reaction type: Michael Reaction
pf
150-157 °C (lit.)
grupo funcional
phosphine oxide
cadeia de caracteres SMILES
O=P(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C18H15OP/c19-20(16-10-4-1-5-11-16,17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
chave InChI
FIQMHBFVRAXMOP-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- As a catalyst in Appel-type chlorination reaction of acyclic primary and secondary alcohols.
- As a catalyst in stereoselective poly and dibromination of α,β-unsaturated esters and β,γ-unsaturated α-ketoester compounds.
- As a promotor in the diastereoselective synthesis of α-ribofuranosides through ribofuranosylation of alcohols with ribofuranosyl iodides.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Chronic 3
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
356.0 °F
Ponto de fulgor (°C)
180 °C
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica