N1909
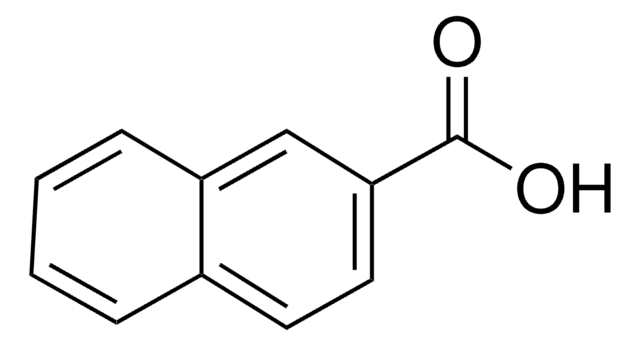
1-Naphthoic acid
96%
Sinônimo(s):
1-Naphthalenecarboxylic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C10H7CO2H
Número CAS:
Peso molecular:
172.18
Beilstein:
1908896
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
96%
Formulário
powder
p.e.
300 °C (lit.)
pf
157-160 °C (lit.)
cadeia de caracteres SMILES
OC(=O)c1cccc2ccccc12
InChI
1S/C11H8O2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,12,13)
chave InChI
LNETULKMXZVUST-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
1-Naphthoic acid can be used as a reactant to prepare:
- Perinaphthenones by dehydrative annulation with alkynes in the presence of rhodium catalyst.
- Isocoumarin derivatives by reacting with 2-butyne via aerobic oxidative cyclization using Rh catalyst.
- N-Methoxy-N-methyl-1-naphthalenecarboxamide (Weinreb amide) by reacting with N,O-dimethylhydroxylamine and phosphorus trichloride.
- 1,4-Dihydro-1-naphthalenecarboxylic acid by Birch reduction.
Outras notas
Remainder 2-naphthoic acid
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
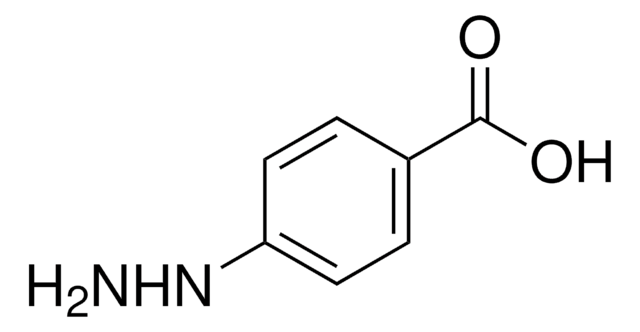
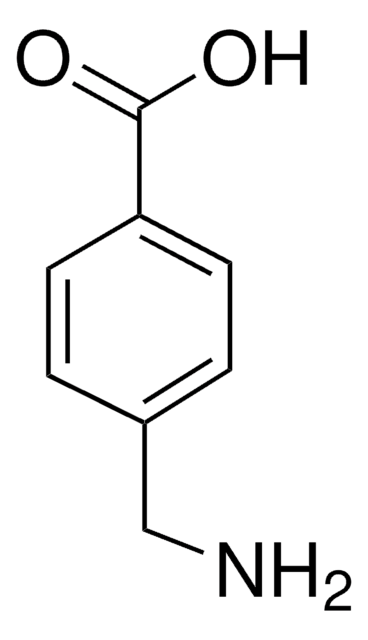
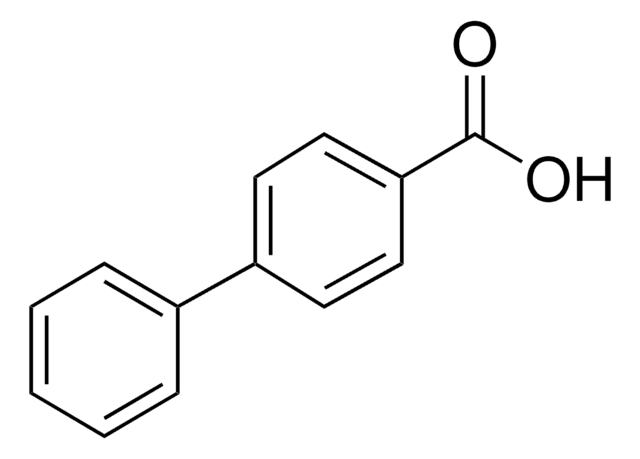
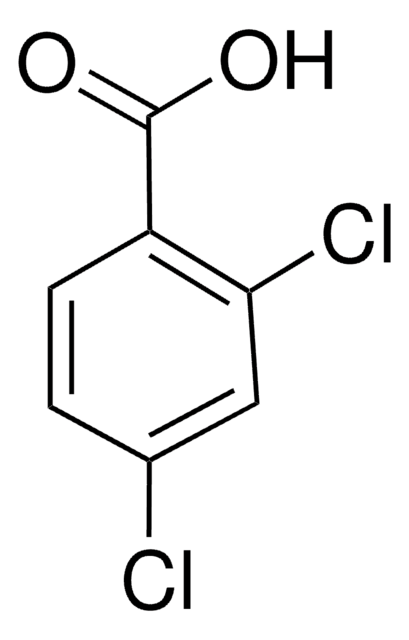
Os clientes também visualizaram
Yu-mei Song et al.
Chemical communications (Cambridge, England), 48(7), 1006-1008 (2011-12-14)
Reversible single-crystal-to-single-crystal transformation (SCSC) was for the first time observed between 4f-based molecular magnets.
Hiromasa Uchiyama et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 43(1-2), 71-77 (2011-04-06)
Spray-dried particles (SDPs) with indomethacin (IND) and alpha-glycosyl transferase-treated stevia (Stevia-G) indicated extremely high dissolution rates and apparent solubility compared to particles of a ground mixture and a physical mixture of IND/Stevia-G. The apparent solubility of IND from SDPs was
Qunfei Zhao et al.
Chemistry & biology, 15(7), 693-705 (2008-07-19)
Azinomycin B is a complex natural product containing densely assembled functionalities with potent antitumor activity. Cloning and sequence analysis of the azi gene cluster revealed an iterative type I polyketide synthase (PKS) gene, five nonribosomal peptide synthetases (NRPSs) genes and
Mutual activation: Suzuki-Miyaura coupling through direct cleavage of the sp2 C-O bond of naphtholate.
Da-Gang Yu et al.
Angewandte Chemie (International ed. in English), 50(31), 7097-7100 (2011-06-29)
Rajesh Sunasee et al.
The Journal of organic chemistry, 73(20), 8016-8020 (2008-09-26)
A method is described for converting tert-butyl benzoates or tert-butyl 1-naphthoates into derivatives having an alkyl or substituted alkyl group in a 1,4-relationship to an alkyl, aryl, alkenyl, or alkynyl group. Key steps in the sequence are (i) addition of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica