D4407
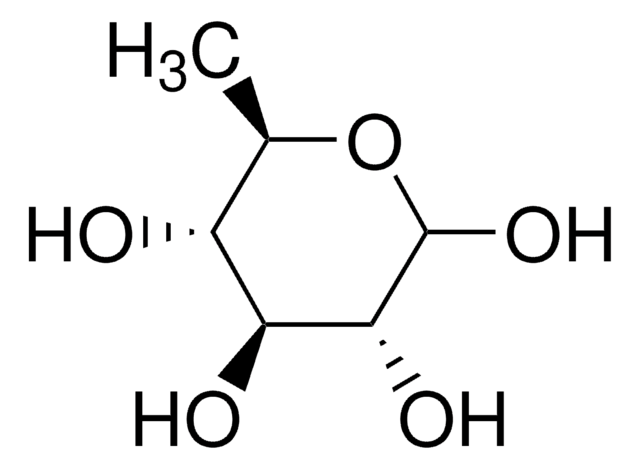
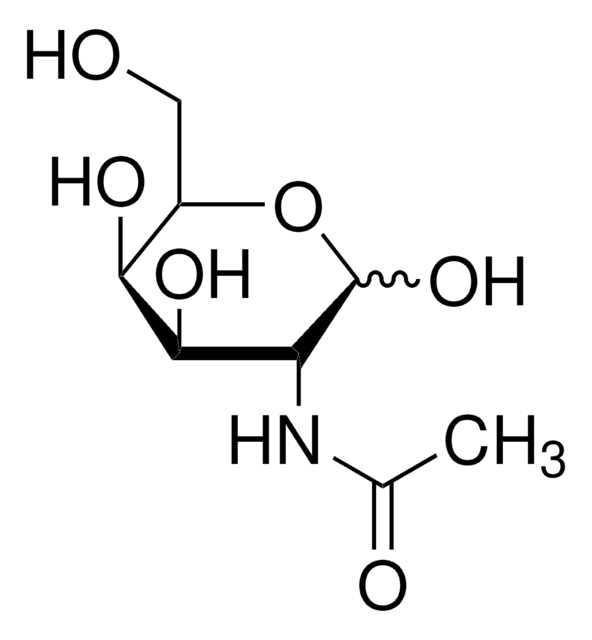
2-Deoxy-D-galactose
98%
Sinônimo(s):
2-Deoxy-D-lyxohexose
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H12O5
Número CAS:
Peso molecular:
164.16
Beilstein:
1723333
Número CE:
Número MDL:
Código UNSPSC:
12352201
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
powder
atividade óptica
[α]20/D +59.7°, c = 2 in H2O
cor
white to off-white
pf
107 - 110 °C ((225 - 230 °F))
107-110 °C (lit.)
cadeia de caracteres SMILES
OC[C@H]1OC(O)C[C@@H](O)[C@H]1O
InChI
1S/C6H12O5/c7-2-4-6(10)3(8)1-5(9)11-4/h3-10H,1-2H2/t3-,4-,5?,6-/m1/s1
chave InChI
PMMURAAUARKVCB-DUVQVXGLSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
2-Deoxy-D-galactose is a glucose analog that shows a wide range of biological activities such as inhibition of glycolysis and thereby tumor growth, interference with the biosynthetic processing of glycoproteins, antiviral activity, and hepatotoxicity. It is being extensively studied as trapping agents for phosphate and uridylate in mammalian cells due to its ability to interfere in the phosphate and nucleotide metabolism.
Aplicação
- FUT1-mediated terminal fucosylation acts as a new target to attenuate renal fibrosis.: This research investigates the role of 2-deoxy-D-galactose in modulating terminal fucosylation processes, revealing potential therapeutic pathways for treating renal fibrosis and enhancing the understanding of kidney disease mechanisms (Luo et al., 2023).
- 2-D-gal Targets Terminal Fucosylation to Inhibit T-cell Response in a Mouse Skin Transplant Model.: Highlights the immunomodulatory potential of 2-deoxy-D-galactose in transplant medicine, showing how it can inhibit T-cell responses and contribute to the success of skin grafts, pointing towards new immunosuppressive treatments (Mao et al., 2023).
- Inhibition of Aberrant α(1,2)-Fucosylation at Ocular Surface Ameliorates Dry Eye Disease.: Explores the therapeutic effects of 2-deoxy-D-galactose in treating dry eye disease by modulating specific fucosylation pathways, potentially opening new avenues for ocular surface treatment strategies (Yoon et al., 2021).
Outras notas
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Gaetano Donofrio et al.
PloS one, 8(1), e52758-e52758 (2013-01-10)
Caprine herpesvirus type 1 (CpHV-1) is an alphaherpesvirus causing genital disease leading to abortion in adult pregnant goats and a systemic disease with high morbility and mortality in kids. Further, Caprine herpesvirus 1 infection represents a valuable large animal model
T Platt
Molecular and cellular biology, 4(5), 994-996 (1984-05-01)
Analysis of 400 independent spontaneous mutations conferring 2-deoxygalactose resistance upon cells constitutive for the galactose pathway suggests that toxicity is due to 2-deoxygalactose-1-phosphate. Selection for and against growth on galactose in the same strain is now possible; application to systems
2-Deoxy-D-galactose impairs the fucosylation of glycoproteins of rat liver and Morris hepatoma.
R Büchsel et al.
European journal of biochemistry, 111(2), 445-453 (1980-10-01)
J A Gorman et al.
Genetics, 129(1), 19-24 (1991-09-01)
A technique which has the potential to allow repeated use of the same selectable marker to create gene disruptions in Candida albicans has been developed. In this approach, originally described for Saccharomyces cerevisiae, the selectable marker is flanked by direct
Raphaël Dutoit et al.
Nucleic acids research, 38(19), e183-e183 (2010-08-13)
Diverse tools are available for performing genetic modifications of microorganisms. However, new methods still need to be developed for performing precise genomic engineering without introducing any undesirable side-alteration. Indeed for functional analyses of genomic elements, as well as for some
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica