D139459
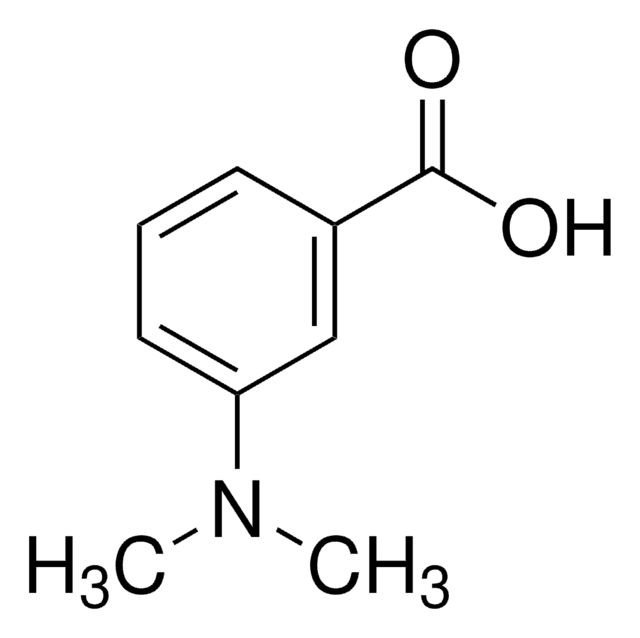
4-(Dimethylamino)benzoic acid
98%, for peptide synthesis
Sinônimo(s):
N,N-Dimethyl-4-aminobenzoic acid, N,N-Dimethyl-p-aminobenzoic acid, p-(Dimethylamino)benzoic acid
About This Item
Produtos recomendados
Nome do produto
4-(Dimethylamino)benzoic acid, 98%
Nível de qualidade
Ensaio
98%
Formulário
powder and chunks
adequação da reação
reaction type: solution phase peptide synthesis
pf
241-243 °C (dec.) (lit.)
aplicação(ões)
peptide synthesis
cadeia de caracteres SMILES
CN(C)c1ccc(cc1)C(O)=O
InChI
1S/C9H11NO2/c1-10(2)8-5-3-7(4-6-8)9(11)12/h3-6H,1-2H3,(H,11,12)
chave InChI
YDIYEOMDOWUDTJ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Global Trade Item Number
| SKU | GTIN |
|---|---|
| D139459-100G | 4061838353726 |
| D139459-25G | 4061833559758 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica