C95501
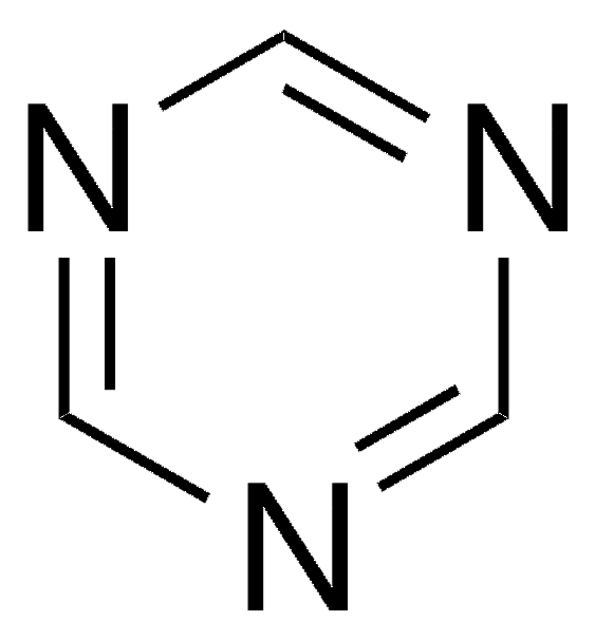
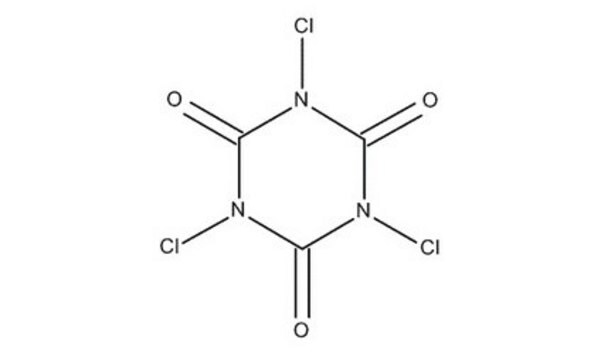
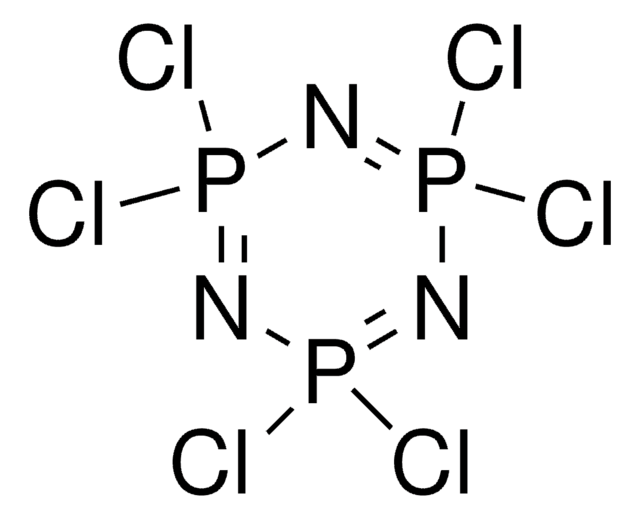
Cyanuric chloride
99%
Sinônimo(s):
2,4,6-Trichloro-1,3,5-triazine
About This Item
Produtos recomendados
densidade de vapor
6.36 (vs air)
Nível de qualidade
pressão de vapor
0.8 mmHg ( 62.2 °C)
Ensaio
99%
forma
powder
pb
190 °C (lit.)
pf
145-147 °C (lit.)
temperatura de armazenamento
2-8°C
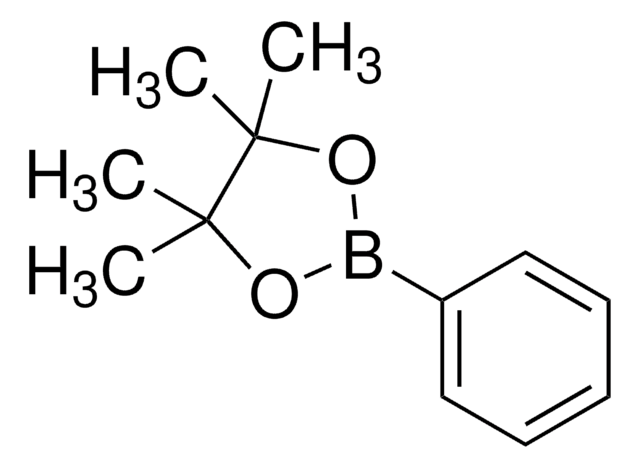
cadeia de caracteres SMILES
Clc1nc(Cl)nc(Cl)n1
InChI
1S/C3Cl3N3/c4-1-7-2(5)9-3(6)8-1
chave InChI
MGNCLNQXLYJVJD-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- In the preparation of acyl azides from carboxylic acids and sodium azide.
- For the conversion of carboxylic acids, N-Boc, N-Cbz, and N-Fmoc amino acids into corresponding alcohols.
- For the conversion of alcohols into the corresponding carbonyl compounds by alternative Swern oxidation reaction.
It can also be employed as a catalyst in the Beckmann rearrangement of ketoximes into amides in the presence of ZnCl2.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A - STOT SE 3
Órgãos-alvo
Respiratory system
Perigos de suplementos
Código de classe de armazenamento
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
>392.0 °F - closed cup
Ponto de fulgor (°C)
> 200 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Collagen molecules play a critical role in tissue architecture and strength, and in cell-matrix interactions as insoluble ligands to regulate the diverse phenotypic activities of cells.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica