930962
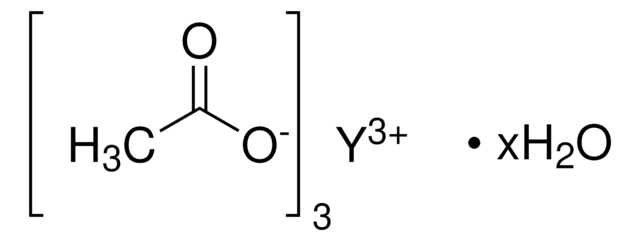
Yttrium(III) acetate tetrahydrate
99.99% trace rare earth metals basis
Sinônimo(s):
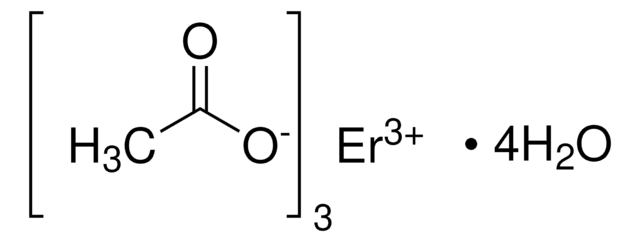
Acetic acid yttrium(3+) salt, Yttrium triacetate
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
99.99% trace rare earth metals basis
Formulário
powder
Impurezas
≤150 ppm trace rare earth metals
≤500 ppm trace metals
pf
350 °C (Decomp.)
solubilidade
H2O: soluble (lit.)
densidade
1.5 g/cm3
Categorias relacionadas
Descrição geral
Aplicação
A major application of high-purity yttrium acetate is in the synthesis of sodium yttrium fluoride (NaYF4) nanoparticles. Typically, in these syntheses, yttrium acetate is mixed with oleic acid in octadecene and heated to form Y(oleate)3, which is reacted with ammonium fluoride and sodium hydroxide in methanol at modest temperatures (e.g. 50 C) to form NaYF4 nanoparticles. This synthesis offers great control over particle size and crystallinity and allows for easy incorporation rare-earth metal dopants.
Lanthanide-doped NaYF4 nanoparticles are one of the most studied materials for up conversion. These nanoparticles, which can convert two photons of near-infrared (NIR) light into visible light, have important in-vivo applications because of the deep tissue penetration abilities of NIR. For example, these nanoparticles have been used for in-vivo Zn2+ optical sensing, in-vivo ratiometric sensing of lymphatic inflammation,, and in-vivo sensing of peroxynitrite.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica