803448
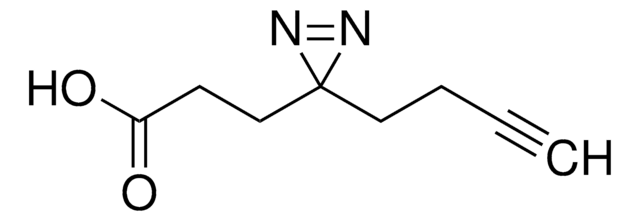
SDAD (NHS-SS-Diazirine) (succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithiopropionate)
About This Item
Produtos recomendados
Ensaio
≥90%
forma
powder
peso molecular
388.46
adequação da reação
reagent type: cross-linking reagent
condição de armazenamento
desiccated
solubilidade
DMSO or DMF: soluble
Condições de expedição
ambient
temperatura de armazenamento
2-8°C
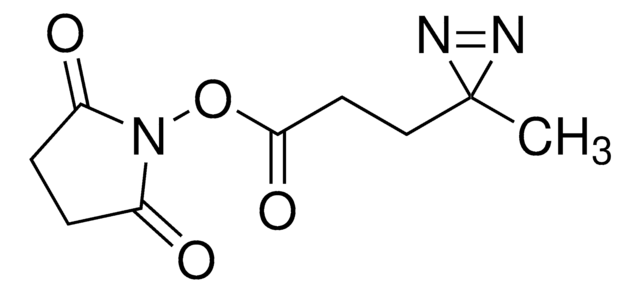
cadeia de caracteres SMILES
CC1(N=N1)CCC(NCCSSCCC(ON2C(CCC2=O)=O)=O)=O
InChI
1S/C14H20N4O5S2/c1-14(16-17-14)6-4-10(19)15-7-9-25-24-8-5-13(22)23-18-11(20)2-3-12(18)21/h2-9H2,1H3,(H,15,19)
chave InChI
NLPWBELUEANJAT-UHFFFAOYSA-N
Descrição geral
Características e benefícios
- Membrane-permeable—suitable for in vivo intracellular protein crosslinking
- Heterobifunctional—NHS ester group reacts with primary amines at pH 7 to 9 to form covalent amide bonds; diazirine (azipentanoate) group reacts efficiently with any amino acid side chain or peptide backbone upon activation with long-wave UV light (330-370 nm)
- Controllable—two-step chemical crosslinking is activated using common laboratory UV lamps
- Easy to use—these crosslinkers are photo-stable under typical laboratory lighting conditions so there is no need to perform experiments in the dark
- Better than aryl azides—the diazirine photoreactive group has better photostability in normal light than phenyl azide groups of traditional photoreactive crosslinkers, yet the diazirine group is more efficiently activated by long-wave UV light
Atenção
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica