791571
Isopropyl p-toluenesulfonate
97%
Sinônimo(s):
Isopropyl p-tosylate
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H14O3S
Número CAS:
Peso molecular:
214.28
Número CE:
Número MDL:
Código UNSPSC:
12352100
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.503
densidade
1.142 g/mL at 25 °C
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
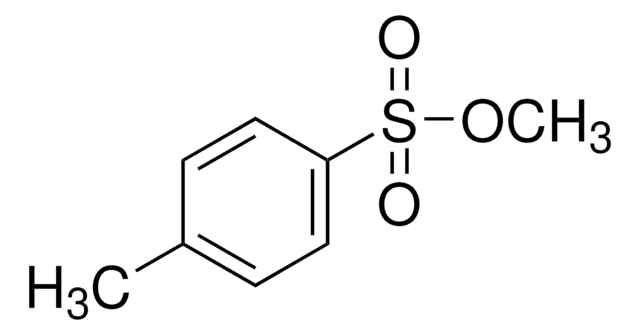
[S](=O)(=O)(Oc1ccc(cc1)C)C(C)C
InChI
1S/C10H14O3S/c1-8(2)14(11,12)13-10-6-4-9(3)5-7-10/h4-8H,1-3H3
chave InChI
MUTOLSSCMLECRU-UHFFFAOYSA-N
Aplicação
Isopropyl p-toluenesulfonate can be used as a reactant:
- For the alkylation of amines in the presence of triethylamine.
- For the O-alkylation of cyclic thiohydroxamic acids in the presence of tetrabutylammonium hydroxide.
- To synthesize 3-methyl-2-phenyl-1-butanol by reacting with styrene in the presence of bromo(2-cyclohexylethyl)magnesium and zirconocene dichloride.
Isopropyl p-toluenesulfonate can be used:
- as a thermal acid generator that synthesizes inherently stretchable p-conjugated polymer
- as a catalyst for the deprotection of PBHEMA [Poly(2-(tert-butoxycarbonyloxy)ethyl methacrylate)] on heating
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Design, Synthesis and Antimicrobial Evaluation of Novel Tricyclic Benzoxazine Fluoroquinolones under Conventional and Microwave Methods
Guruswamy B, et al.
Journal of Heterocyclic Chemistry, 52, 532-538 (2015)
B Y P Tay et al.
International journal of cosmetic science, 38(6), 627-633 (2016-05-14)
Isopropyl p-toluenesulfonate (IPTS) is a potentially genotoxic by-product formed during the esterification of palm oil-based palmitic and palm kernel oil-based myristic acid with isopropanol to produce isopropyl palmitate or isopropyl myristate. There are no methods described for the analysis of
J de Armas et al.
Organic letters, 3(13), 2097-2100 (2001-06-22)
[reaction: see structure] The first examples of efficient electrophilic Zr-catalyzed carbomagnesations are disclosed, where in contrast to previous catalytic carbomagnesations the alkyl moiety of the electrophile is transferred (vs that of the Grignard reagent). The identity of the Grignard reagent
Satsuki Chikura et al.
Mutation research, 811, 110-116 (2016-12-10)
As part of a collaborative study in the Mammalian Mutagenicity Study group of the Japanese Environmental Mutagen Society, we evaluated the in vivo mutagenicity of isopropyl p-toluenesulfonate (IPTS) using a peripheral blood Pig-a assay in rats. Pig-a mutant frequency (MF)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 791571-1G | 4061832949918 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica