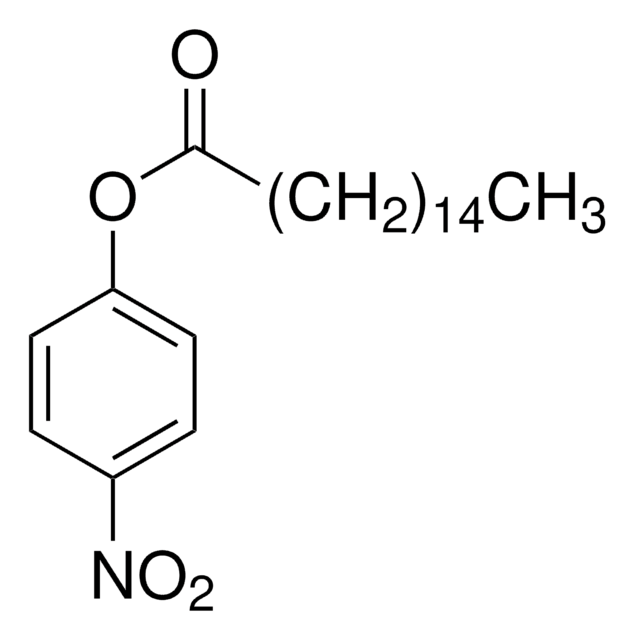
701122
Iron(III) chloride
sublimed grade, ≥99.9% trace metals basis
Sinônimo(s):
Ferric chloride, Iron trichloride, Molysite
About This Item
Produtos recomendados
grau
sublimed grade
Nível de qualidade
densidade de vapor
5.61 (vs air)
pressão de vapor
1 mmHg ( 194 °C)
Ensaio
≥99.9% trace metals basis
Formulário
powder or crystals
adequação da reação
reagent type: catalyst
core: iron
características do produto alternativo mais ecológico
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
técnica(s)
cell culture | mammalian: suitable
Impurezas
≤1000.0 ppm Trace Metal Analysis
pf
304 °C (lit.)
aplicação(ões)
battery manufacturing
categoria alternativa mais ecológica
cadeia de caracteres SMILES
Cl[Fe](Cl)Cl
InChI
1S/3ClH.Fe/h3*1H;/q;;;+3/p-3
chave InChI
RBTARNINKXHZNM-UHFFFAOYSA-K
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- As a precursor to prepare Fe(III)-chlorophyll complex. Fe(III) enhances the performance of chlorophyll as a dye sensitizer in DSSCs by forming complex compounds with metal-ligand charge transfer properties, leading to increased efficiency and improved paramagnetic properties.
- As a catalyst /modifying agent to prepare high performance porous carbon for lithium-ion battery anodes. The addition of FeCl3 enhances the graphitization of porous carbon without significantly affecting the layer spacing and also the specific surface area and pore volume of porous carbon.
- As a co-catalyst to fabricate liquid catalyzed fuel cell (LCFC) for direct conversion from carbohydrates to electricity. It helps to improve the hydrolysis of carbohydrate and enhances the electron transfer from carbohydrates to anode.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Irrit. 2
Código de classe de armazenamento
8B - Non-combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Professor Randal Lee (University of Houston, USA) discusses design considerations for iron oxide magnetic nanospheres and nanocubes used for biosensing, including synthetic procedures, size, and shape. The effects of these variables are discussed for various volumetric-based and surface-based detection schemes.
Lithium-Ion Battery Performance: Dependence on Material Synthesis and Post‑Treatment Methods
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 701122-1G | 4061832796062 |
| 701122-25G | 4061832796079 |
| 701122-5G | 4061832796109 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica