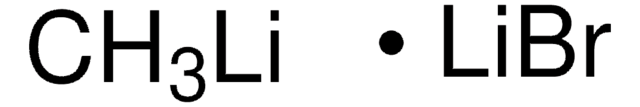693537
R-MOP
≥94%
Sinônimo(s):
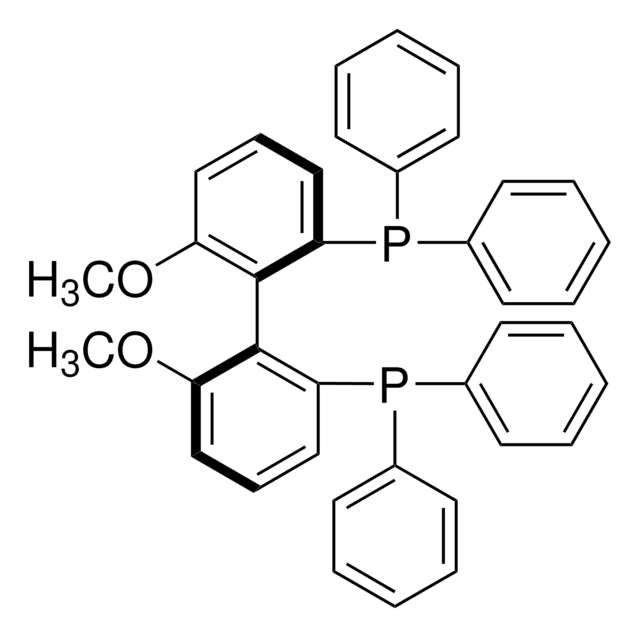
(R)-(+)-2-(Diphenylphosphino)-2′-methoxy-1,1′-binaphthyl
About This Item
Produtos recomendados
Ensaio
≥94%
forma
solid
atividade óptica
[α]20/D +94°, c = 0.5 in chloroform
cadeia de caracteres SMILES
COc1ccc2ccccc2c1-c3c(ccc4ccccc34)P(c5ccccc5)c6ccccc6
InChI
1S/C33H25OP/c1-34-30-22-20-24-12-8-10-18-28(24)32(30)33-29-19-11-9-13-25(29)21-23-31(33)35(26-14-4-2-5-15-26)27-16-6-3-7-17-27/h2-23H,1H3
chave InChI
KRWTWSSMURUMDE-UHFFFAOYSA-N
Descrição geral
Aplicação
Ligand used in palladium-catalyzed asymmetric hydrosilylation of olefins, palladium-catalyzed reduction of allylic esters, rhodium-catalyzed asymmetric addition reactions, and asymmetric amination reactions catalyzed by copper(I) complexes.
Informações legais
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
In the last few years, a number of chiral diene ligands have emerged for a variety of asymmetric transformations.1 This powerful new method has proven to be an efficient way for the construction of enantioenriched compounds from achiral substrates.
Hydrogenation, Asymmetric Catalysis, Binap, SEGPHOS®, Aldol reaction, Alkenylation, Arylation, Mannich reaction, Fluorination, Michael addition, Hydrosilylation, Cycloaddition, Takasago
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![(S)-(+)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4- a′]dinaphthalen-4-yl)dimethylamine 97%](/deepweb/assets/sigmaaldrich/product/structures/400/008/628143de-3954-440a-ba9c-4c0ff8e44663/640/628143de-3954-440a-ba9c-4c0ff8e44663.png)

![(S)-(+)-N-(3,5-Dioxa-4-phosphacyclohepta[2,1-a;3,4-a′]dinaphthalen-4-yl)-dibenzo[b,f]azepine ≥95% (elemental analysis)](/deepweb/assets/sigmaaldrich/product/structures/575/489/d54360f9-5a59-43f2-bc44-42f5fa92b588/640/d54360f9-5a59-43f2-bc44-42f5fa92b588.png)
![(R)-(–)-4,12-Bis(diphenylphosphino)-[2.2]-paracyclophane 96%](/deepweb/assets/sigmaaldrich/product/structures/131/143/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2/640/7e18cd49-a90e-4d89-a189-4f37ad9e6cd2.png)