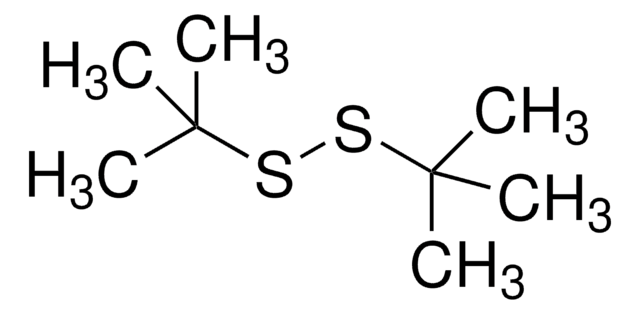
471569
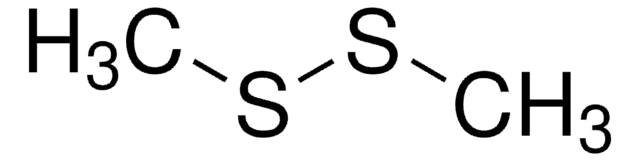
Dimethyl disulfide
≥99.0%
Sinônimo(s):
DMDS, Methyl disulfide
About This Item
Produtos recomendados
densidade de vapor
3.24 (vs air)
Nível de qualidade
pressão de vapor
22 mmHg ( 20 °C)
Ensaio
≥99.0%
temperatura de autoignição
>572 °F
Lim. expl.
16 %
índice de refração
n20/D 1.525 (lit.)
p.e.
109 °C (lit.)
pf
−85 °C (lit.)
densidade
1.046 g/mL at 25 °C (lit.)
grupo funcional
disulfide
cadeia de caracteres SMILES
CSSC
InChI
1S/C2H6S2/c1-3-4-2/h1-2H3
chave InChI
WQOXQRCZOLPYPM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Sens. 1 - STOT SE 1 Inhalation - STOT SE 3
Órgãos-alvo
Central nervous system, Upper respiratory tract
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
59.0 °F - closed cup
Ponto de fulgor (°C)
15 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
as thiolate at the bridge site
Protocolos
Separation of 4-Methyl-2-pentanone; Dimethyl disulfide; Hexanal; 3-Methylpentane; Acetone
Separation of Sulfur dioxide; Hydrogen sulfide; Carbonyl sulfide; Methanethiol; Ethanethiol; Dimethyl disulfide; Carbon disulfide
Separation of 2-Ethyl-3-methylpyrazine; 1-Methylpyrrole; 2,3-Dimethylpyrazine; 2,5-Dimethylpyrazine; 2-Ethylpyrazine, ≥98%, FG; 2,3-Diethylpyrazine; 2-Methylpyrazine; Carbon disulfide; Dimethyl disulfide; 2,6-Dimethylpyrazine
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica