431362
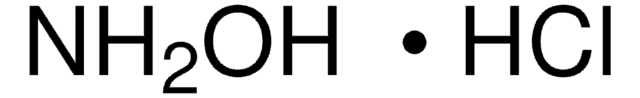
Hidroxilamina
99.999% trace metals basis
Sinônimo(s):
Cloreto de hidroxilamônio
About This Item
Produtos recomendados
grau
ACS reagent (specifications)
Ensaio
99.999% trace metals basis
Formulário
crystals
Impurezas
≤0.005% S compounds
≤0.25 meq/g Titr. free acid
<10 ppm total metallic impurities
resíduo de ignição
≤0.05%
pH
2.5-3.5 (20 °C, 50 g/L)
pf
155-157 °C (dec.) (lit.)
densidade
1.67 g/mL at 25 °C (lit.)
traços de cátion
Fe: ≤5 ppm
NH4+:, passes test
heavy metals: ≤5 ppm
cadeia de caracteres SMILES
Cl.NO
InChI
1S/ClH.H3NO/c;1-2/h1H;2H,1H2
chave InChI
WTDHULULXKLSOZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Organosilane amines as potent inhibitors and structural probes of influenza A virus M2 proton channel
- Lamellarin D analogues as inibitors of topoisomerase I and potential antitumor agents
- Azapeptide tocolytic agents as inhibitors of prostaglandin F2a receptor for preventing preterm labor
- Thiazolidinones spiro-fused to indolin-2-ones as potent and selective inhibitors of Mycobacterium tuberculosis protein tyrosine phosphatase B
- Orally bioavailable quinoline-based antidiabetic dipeptidyl peptidase IV inhibitors targeting Lys554
- Pyrimidine nucleoside derivatives with nitric oxide donors as antiviral agents
- Benzyladenosine compounds targeting adenosine A2A receptor and adenosine transporter for neuroprotection
- Naphthol derivatives as inhibitors of the vanilloid receptor TRPV1 with improved potency in rat cystometry models of urinary incontinence
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 2 - Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT RE 2 Oral
Órgãos-alvo
spleen
Código de classe de armazenamento
4.1A - Other explosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica