About This Item
Fórmula linear:
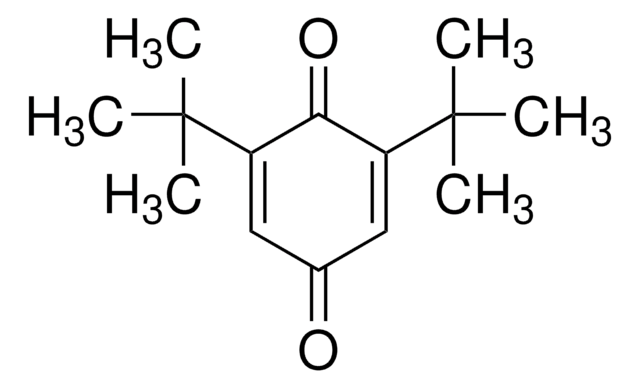
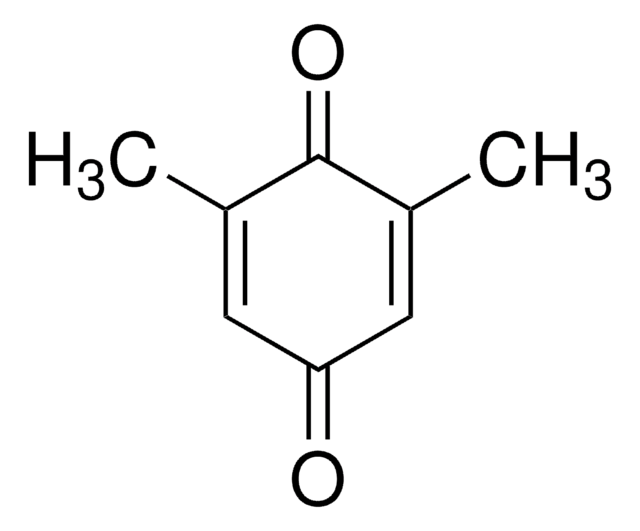
(CH3)3CC6H3(=O)2
Número CAS:
Peso molecular:
164.20
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
Formulário
solid
pf
54-58 °C (lit.)
grupo funcional
ketone
cadeia de caracteres SMILES
CC(C)(C)C1=CC(=O)C=CC1=O
InChI
1S/C10H12O2/c1-10(2,3)8-6-7(11)4-5-9(8)12/h4-6H,1-3H3
chave InChI
NCCTVAJNFXYWTM-UHFFFAOYSA-N
Descrição geral
2-tert-Butyl-1,4-benzoquinone (TBQ, TBBQ, tBQ, BuBQ, BQ , tert-butyl-p-quinone) is a 1,4-benzoquinone derivative. It is a major metabolite of the food additive, butylated hydroxyanisole (BHA). TBQ is reported to be strongly cytotoxic in human monocytic leukemia U937 cells. TBQ is an oxidation product of 2-tert-butylhydroquinone (TBHQ). Studies confirm that TBQ induces apoptosis and cell proliferation inhibition in chronic myelogenous leukemia (CML) cells. Its binding interactions with lysozyme has been examined and found to be intermediate between BHA and TBHQ. It has been reported to be synthesized by the titanium superoxide catalyzed oxidation of 2-tert-butylphenol using aq. 30% H2O2. TBQ is one of the main neoformed compounds from TBHQ decomposition in PLA-TBHQ film (Poly lactic acid).
Aplicação
2-tert-Butyl-1,4-benzoquinone may be used in the synthesis of azatrioxa[8]circulene.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
2-tert-butyl-1, 4-benzoquinone Induces Apoptosis in Chronic Myeloid Leukemia Cells Resistant to Imatinib via Inducing Caspase-Dependent Bcr-Abl Downregulation.
Shi X, et al.
Medicinal Chemistry, 4, 784-790 (2014)
R Kahl et al.
Toxicology, 59(2), 179-194 (1989-12-01)
The synthetic antioxidant butylated hydroxyanisole (BHA) stimulates superoxide formation in rat liver microsomes up to 10-fold. This stimulation is prevented by the monooxygenase inhibitor metyrapone and does not occur when NADH is consumed instead of NADPH indicating that metabolic activation
W H Kalus et al.
Environmental health perspectives, 102(1), 96-99 (1994-01-01)
We examined t-butylhydroquinone (t-BHQ) and t-butylquinone (t-BuQ), two of the major microsomal metabolites of the synthetic antioxidant butylated hydroxyanisole (BHA), for their ability to react with the xenobiotic arylamines aniline and N-methylaniline. A number of substances were isolated by thin-layer
Azatrioxa [8] circulenes: Planar Anti-Aromatic Cyclooctatetraenes.
Nielsen CB, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(12), 3898-3904 (2013)
P A Schilderman et al.
Carcinogenesis, 16(3), 507-512 (1995-03-01)
The food additive butylated hydroxyanisole (BHA) has been shown to induce gastrointestinal hyperplasia in rodents by an unknown mechanism. The relevance of this observation for human risk assessment is not clear. We therefore analysed the effect of BHA and its
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica