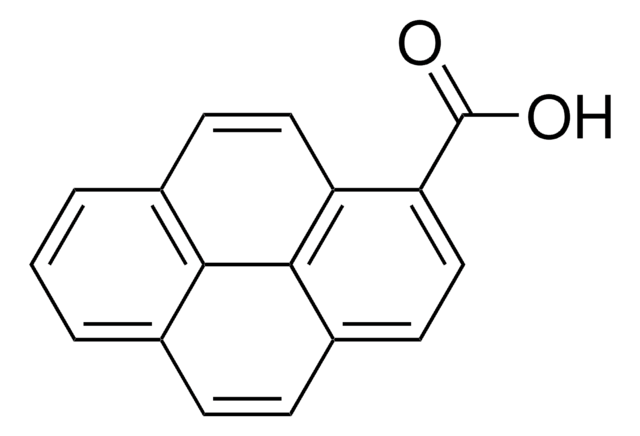
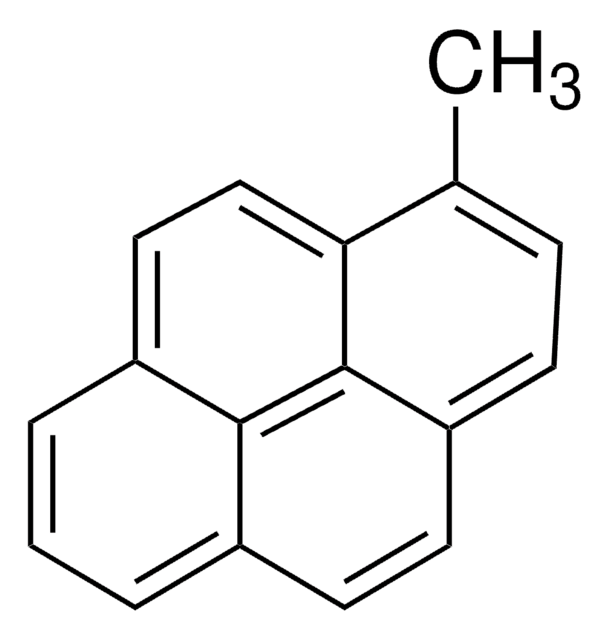
389439
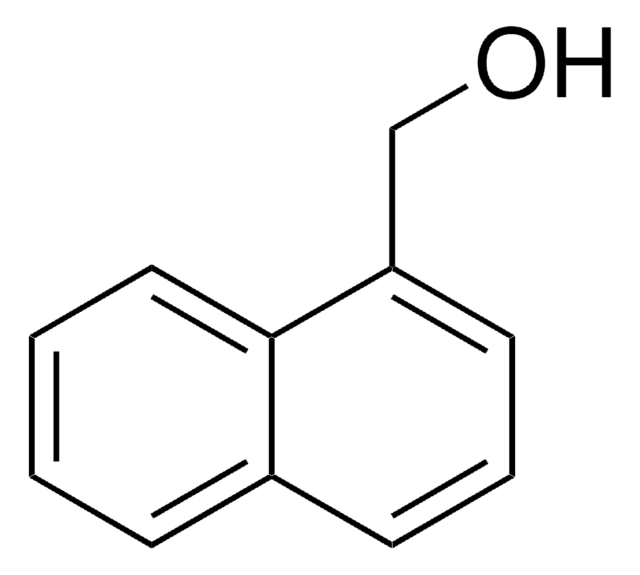
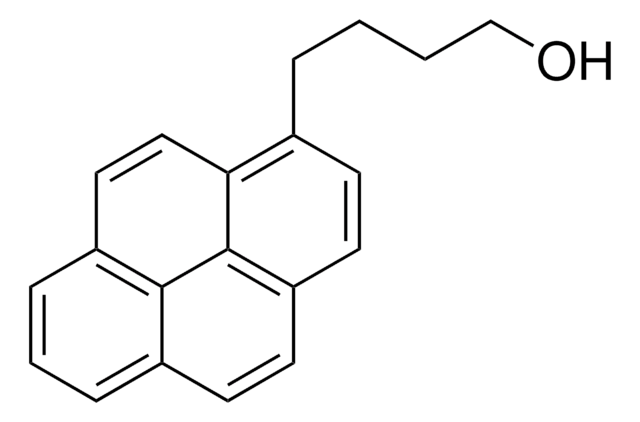
1-Pyrenemethanol
98%
Sinônimo(s):
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C17H12O
Número CAS:
Peso molecular:
232.28
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
forma
solid
pf
123-126 °C (lit.)
grupo funcional
hydroxyl
cadeia de caracteres SMILES
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
chave InChI
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Aplicação
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
H Glatt et al.
Carcinogenesis, 14(4), 599-602 (1993-04-01)
1-Hydroxymethylpyrene (HMP), a primary benzylic alcohol, and 4H-cyclopenta[def]chrysen-4-ol (OH-CPC), a secondary benzylic alcohol, were investigated for mutagenicity in Salmonella typhimurium (reversion of the his- strain TA98) in the presence of various xenobiotic-metabolizing systems. In the direct test, HMP was inactive
Encapsulation of functional moieties within branched star polymers: effect of chain length and solvent on site isolation.
Hecht S, et al.
Journal of the American Chemical Society, 123(1), 18-25 (2001)
Walter Meinl et al.
Pharmacogenetics, 12(9), 677-689 (2002-12-05)
Various enzymatically formed sulfuric acid esters are chemically reactive and mutagenic. This metabolic activation pathway is not detected in standard in-vitro mutagenicity test systems. We describe the construction of Salmonella typhimurium TA1538-derived strains expressing alloenzymes *1, *2, *3, *5, *6
Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst.
Ji H, et al.
Tetrahedron Letters, 43(40), 7179-7183 (2002)
H Glatt et al.
Chemico-biological interactions, 109(1-3), 195-219 (1998-05-05)
Sulfation is a common final step in the biotransformation of xenobiotics and is traditionally associated with inactivation. However, the sulfate group is electron-withdrawing and may be cleaved off heterolytically in some molecules leading to electrophilic cations which may form adducts
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)