About This Item
Fórmula empírica (Notação de Hill):
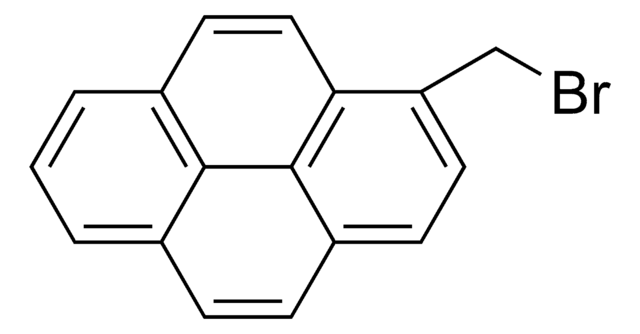
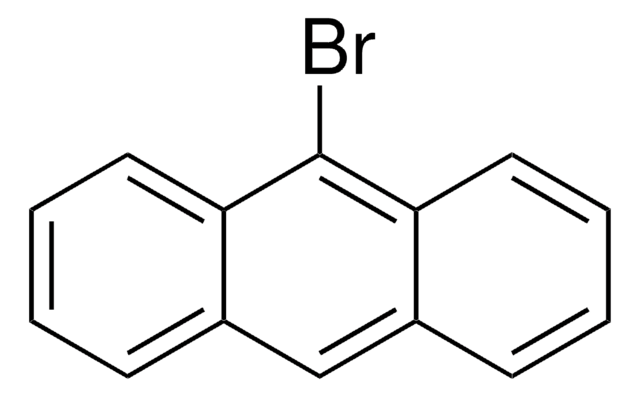
C16H9Br
Número CAS:
Peso molecular:
281.15
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
96%
Formulário
powder
pf
102-105 °C (lit.)
grupo funcional
bromo
cadeia de caracteres SMILES
Brc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C16H9Br/c17-14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9H
chave InChI
HYGLETVERPVXOS-UHFFFAOYSA-N
Descrição geral
1-Bromopyrene, a polycyclic aromatic hydrocarbon (PAH), is a mono bromo substituted pyrene derivative. Its synthesis has been reported. Its gas phase UV-absorption spectra in the UV wavelength range at elevated temperature has been studied. Electron affinitiy (EA) of 1-bromopyrene has been investigated using electron attachment desorption chemical ionization mass spectrometry (DCI-MS) and triple quadrupole tandem mass spectrometry. It participates in the synthesis of novel ruthenium (II) bipyridine or terpyridine complexes bearing pyrene moiety. The reaction of 1-bromopyrene cation radical with water in acetonitrile has been analyzed using the electron transfer stopped-flow (ETSF) method.
Aplicação
1-Bromopyrene is suitable reagent used in the comparative study of effect of substituents of some pyrene derivatives in inducing phototoxicity, DNA damage and repair in human skin keratinocytes and light-induced lipid peroxidation in methanol. It is suitable reagent used in the study to investigate the UV photon-assisted thermal decomposition of PAHs at elevated temperature.
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
1-Bromopyrene may be used as a standard to compare its spectral properties with that of pyrene based fluorescence probe. It may be used to study the effects of the addition of halogen hetero-atoms on the vapor pressures and thermodynamics of polycyclic aromatic hydrocarbons.
It may be used in the synthesis of the following:
- 2-methyl-4-pyren-1-yl-but-3-yn-2-ol
- 1-ethynylpyrene
- silsesquioxane (SSQ) based hybrid
- ruthenium nanoparticles functionalized with pyrene moiety
- mono- and di-pyrenyl perfluoroalkanes
- oligo(1-bromopyrene)(OBrP) films
- dinitropyrene-derived DNA adduct
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Electron affinities of polycyclic aromatic hydrocarbons determined by the kinetic method.
Chen G and Cooks RG.
Journal of Mass Spectrometry : Jms, 30(8), 1167-1173 (1995)
Pyrene-functionalized ruthenium nanoparticles: novel fluorescence characteristics from intraparticle extended conjugation.
Chen W, et al.
The Journal of Physical Chemistry C, 113(39), 16988-16995 (2009)
Novel fluorescence probe based on pyrene and piperazine; spectral properties in solution and in polymer matrices.
Hrdlovic P et al.
Journal of Photochemistry and Photobiology A: Chemistry, 163(1), 289-296 (2004)
Tracie Perkins Fullove et al.
Toxicology research, 2(3), 193-199 (2014-06-06)
Polycyclic aromatic hydrocarbons (PAHs), a class of mutagenic environmental contaminants, insert toxicity through both metabolic activation and light irradiation. Pyrene, one of the most widely studied PAHs, along with its mono-substituted derivatives, 1-amino, 1-bromo, 1-hydroxy, and 1-nitropyrene, were chosen to
UV photon-assisted incineration of polycyclic aromatic hydrocarbons at elevated temperatures between 150 and 800?C.
Thony A and Rossi MJ.
Journal of Photochemistry and Photobiology A: Chemistry, 109(3), 267-280 (1997)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica