About This Item
Fórmula linear:
(CH3)3CCH(C6H5)OH
Número CAS:
Peso molecular:
164.24
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
99%
p.e.
90 °C/5 mmHg (lit.)
pf
43-45 °C (lit.)
grupo funcional
hydroxyl
phenyl
cadeia de caracteres SMILES
CC(C)(C)C(O)c1ccccc1
InChI
1S/C11H16O/c1-11(2,3)10(12)9-7-5-4-6-8-9/h4-8,10,12H,1-3H3
chave InChI
YBVRFTBNIZWMSK-UHFFFAOYSA-N
Descrição geral
Raney nickel and Raney cobalt catalyzed transfer hydrogenolysis of 2,2-dimethyl-1-phenyl-1-propanol has been reported. Kinetic resolution of 2,2-dimethyl-1-phenyl-1-propanol using a lead dioxide anode modified with poly-S-valine grafted on to a polypyrrole film has been reported. An efficient Cu(I)-catalyzed oxidation of 2,2-dimethyl-1-phenyl-1-propanol with di-tert-butyldiaziridinone as oxidant under mild conditions has been investigated.
Aplicação
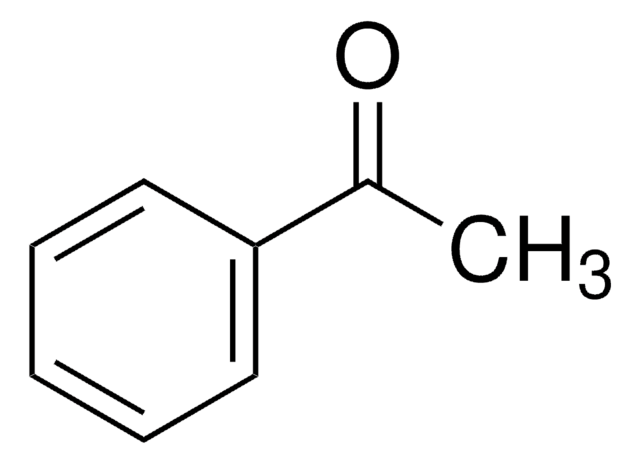
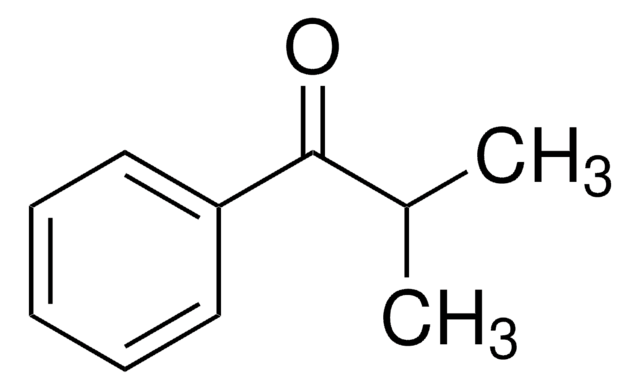
2,2-Dimethyl-1-phenyl-1-propanol was used in the preparation of 2,2-dimethylpropiophenone.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
206.6 °F - closed cup
Ponto de fulgor (°C)
97.00 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Poly (pyrroles) containing chiral side chains: effect of substituents on the chiral recognition in the doped as well as in the undoped state of the polymer film.
Pleus S and Schulte B.
Journal of Solid State Electrochemistry, 5(7-8), 522-520 (2001)
Brant Landers et al.
The Journal of organic chemistry, 76(5), 1390-1397 (2011-01-22)
The use of commercially available (SIPr)Pd(cinnamyl)Cl (SIPr = 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene) as a precatalyst for the anaerobic oxidation of secondary alcohols is described. The use of this complex allows for a drastic reduction in the reaction times and catalyst loading when compared
Transfer hydrogenolysis of aromatic alcohols using Raney catalysts and 2-propanol.
Gross BH, et al.
Applied Catalysis A: General, 219(1), 281-289 (2001)
Yingguang Zhu et al.
Organic letters, 15(5), 992-995 (2013-02-19)
A novel and efficient Cu(I)-catalyzed oxidation of alcohols has been achieved with di-tert-butyldiaziridinone as the oxidant under mild conditions. A wide variety of primary and secondary alcohols with various functional groups can be oxidized to aldehydes and ketones in high
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica