330469
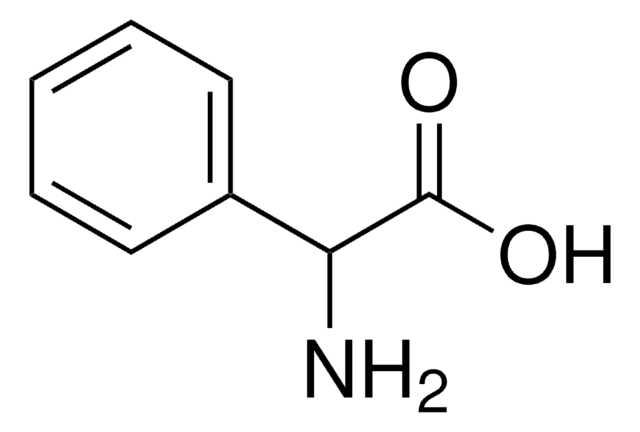
N-Phenylglycine
97%
Sinônimo(s):
(Phenylamino)acetic acid, Anilinoacetic acid
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
C6H5NHCH2COOH
Número CAS:
Peso molecular:
151.16
Beilstein:
509838
Número CE:
Número MDL:
Código UNSPSC:
12352209
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
adequação da reação
reaction type: solution phase peptide synthesis
pf
121-123 °C (lit.)
aplicação(ões)
peptide synthesis
cadeia de caracteres SMILES
OC(=O)CNc1ccccc1
InChI
1S/C8H9NO2/c10-8(11)6-9-7-4-2-1-3-5-7/h1-5,9H,6H2,(H,10,11)
chave InChI
NPKSPKHJBVJUKB-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
J M Janusz et al.
Journal of medicinal chemistry, 33(3), 1052-1061 (1990-03-01)
Twenty esters of L-aspartyl-D-phenylglycine, as well as two substituted analogues, an o-fluoro and a p-hydroxy-phenylglycine ester, were prepared. The L-aspartyl-D-phenylglycine (-)-alpha- and (+)-beta-fenchyl esters had the highest sweetness potency at 1200 and 3700 times that of sucrose, respectively. The high
G E Schumacher et al.
Journal of dental research, 76(1), 602-609 (1997-01-01)
Effective composite-to-dentin bonding has been achieved by the sequential use of dilute aqueous nitric acid (HNO3) and acetone solutions of N-phenylglycine and a carboxylic acid monomer, e.g., p-PMDM. Both the HNO3 pre-treatment and the surface-initiated polymerization that results from reaction
L E Wolinsky et al.
Journal of dental research, 72(1), 72-77 (1993-01-01)
The purpose of the present investigation was to determine whether high-resolution carbon-13 nuclear magnetic resonance could be utilized for detection of ionic bonding interactions of NPG and NPG-GMA with selected inorganic cations. The C1-carbonyl carbon of NPG and NPG-GMA were
N J Miniotis et al.
Journal of dental research, 72(6), 1045-1049 (1993-06-01)
This study evaluated and compared the contributions to dentin adhesive bonding of three N-phenylglycine analogues with electron-withdrawing substituents on the aromatic ring. These electron-deficient "N-compounds" included: N-(4-chlorophenyl)-glycine (NCPG), N-methyl-N-(4-chlorophenyl)-glycine (NMNCPG), and N-(3,4-dichlorophenyl)-glycine (NDCPG). An experimental three-step dentin-bonding protocol that consisted
G Kato et al.
Dental materials : official publication of the Academy of Dental Materials, 14(5), 347-352 (1999-06-24)
The purpose of this study was to investigate the influence of remaining non-resin-impregnated, phosphoric acid demineralized dentin upon the long-term durability of specimens that were wet-bonded to bovine dentin substrates. Prepared bovine dentin samples were etched with 65% phosphoric acid
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica