About This Item
Fórmula linear:
C6F5NO2
Número CAS:
Peso molecular:
213.06
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
forma
liquid
índice de refração
n20/D 1.447 (lit.)
pb
158-161 °C (lit.)
densidade
1.656 g/mL at 25 °C (lit.)
grupo funcional
fluoro
nitro
cadeia de caracteres SMILES
[O-][N+](=O)c1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F5NO2/c7-1-2(8)4(10)6(12(13)14)5(11)3(1)9
chave InChI
INUOFQAJCYUOJR-UHFFFAOYSA-N
Descrição geral
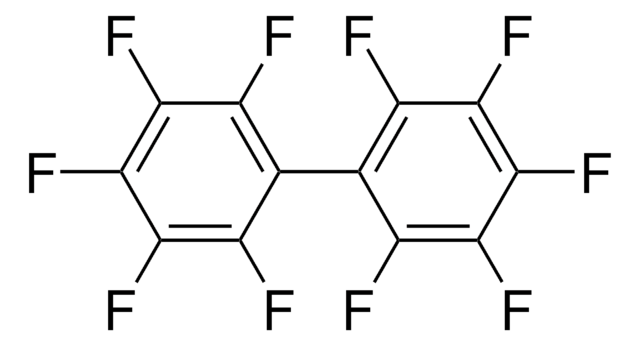
Electron attachment to pentafluoronitrobenzene has been studied in the energy range 0-16eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. The electroreduction of pentafluoronitrobenzene in dimethylformamide solution results in the formation of the dimer, octafluoro-4,4′-dinitro-biphenyl.
Aplicação
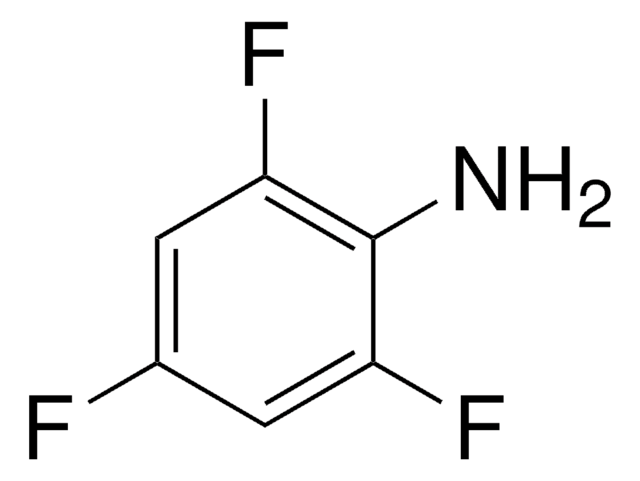
Pentafluoronitrobenzene has been used in the preparation of p-azidotetrafluoroaniline, a new photoaffinity reagent.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
195.8 °F - closed cup
Ponto de fulgor (°C)
91 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Judith Langer et al.
Physical chemistry chemical physics : PCCP, 10(11), 1523-1531 (2008-03-11)
Electron attachment to pentafluorobenzonitrile (C(6)F(5)CN) and pentafluoronitrobenzene (C(6)F(5)NO(2)) is studied in the energy range 0-16 eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. We find that pentafluoronitrobenzene exclusively generates fragment anions via
Voltammetry under high mass transport conditions. The application of the high speed channel electrode to the reduction of pentafluoronitrobenzene.
Coles BA, et al.
Journal of Electroanalytical Chemistry, 411(1), 121-127 (1996)
K A Chehade et al.
The Journal of organic chemistry, 65(16), 4949-4953 (2000-08-24)
p-Azidotetrafluoroaniline (1) was synthesized in 65-73% yield by two different methods employing a stable carbamate intermediate. The first method trapped the intermediate isocyanate generated via a modified Curtius rearrangement with 2-methyl-2-propanol or 2-(trimethylsilyl)ethanol to form the stable carbamates 2d and
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica


![[Bis(trifluoroacetoxy)iodo]benzene 97%](/deepweb/assets/sigmaaldrich/product/structures/238/293/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4/640/71fcde9a-4afb-4cf5-9c22-8d8d68bf1ba4.png)