247820
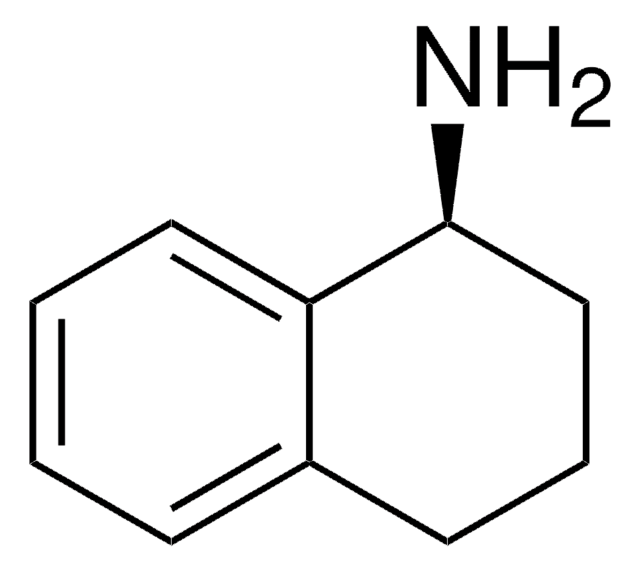
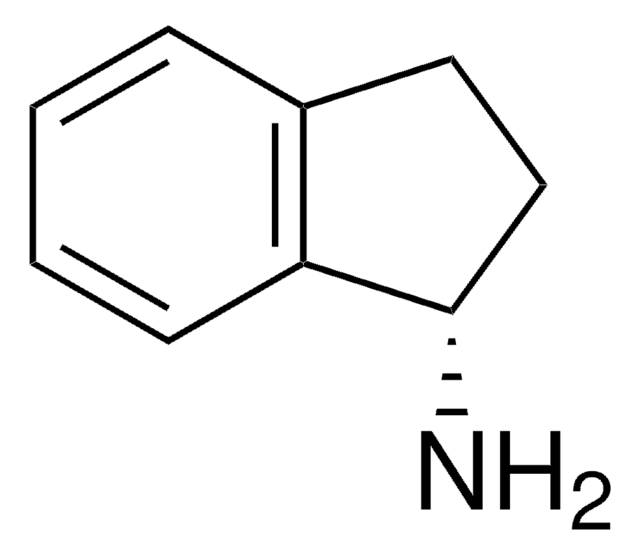
1,2,3,4-Tetrahydro-1-naphthylamine
97%
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C10H13N
Número CAS:
Peso molecular:
147.22
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
liquid
índice de refração
n20/D 1.562 (lit.)
p.e.
246-247 °C/714 mmHg (lit.)
densidade
1.026 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
NC1CCCc2ccccc12
InChI
1S/C10H13N/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6,10H,3,5,7,11H2
chave InChI
JRZGPXSSNPTNMA-UHFFFAOYSA-N
Descrição geral
(R)-1,2,3,4-Tetrahydro-1-naphthylamine is an efficient reagent for iodocyclization of 4-aryl-4-pentenoic acids.
Aplicação
1,2,3,4-Tetrahydro-1-naphthylamine has been used in the preparation of new chiral phosphine-aminophosphine ligands.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F - closed cup
Ponto de fulgor (°C)
113 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Modular Phosphine-Aminophosphine Ligands Based on Chiral 1, 2, 3, 4-Tetrahydro-1-naphthylamine Backbone: A New Class of Practical Ligands for Enantioselective Hydrogenations.
Qiu M, et al.
Advanced Synthesis & Catalysis, 350(17), 2683-2689 (2008)
Jürgen Haas et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 11(19), 5777-5785 (2005-07-23)
Lactonizations are important steps in many synthetic sequences. Substrate-controlled reactions that use chiral auxiliaries or chiral alkenes have already been studied in depth. This study focuses on stereoselective reagent-controlled iodolactonizations, by application of a new method that uses complexes of
J S Shin et al.
Biotechnology and bioengineering, 73(3), 179-187 (2001-03-21)
A kinetic resolution process for the production of chiral amines was developed using an enzyme-membrane reactor (EMR) and a hollow-fiber membrane contactor with (S)-specific omega-transaminases (omega-TA) from Vibrio fluvialis JS17 and Bacillus thuringiensis JS64. The substrate solution containing racemic amine
Noelia Madroñal et al.
Nature communications, 7, 10923-10923 (2016-03-19)
The hippocampus is critical for the acquisition and retrieval of episodic and contextual memories. Lesions of the dentate gyrus, a principal input of the hippocampus, block memory acquisition, but it remains unclear whether this region also plays a role in
Effects of isomers of apomorphines on dopamine receptors in striatal and limbic tissue of rat brain.
N S Kula et al.
Life sciences, 37(11), 1051-1057 (1985-09-16)
The optical isomers of apomorphine (APO) and N-propylnorapomorphine (NPA) were interacted with three biochemical indices of dopamine (DA) receptors in extrapyramidal and limbic preparations of rat brain tissue. There were consistent isomeric preferences for the R(-) configuration of both DA
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica