241636
2,2′-Bithiophene
99%
Sinônimo(s):
2,2′-Bithienyl, 2,2′-Dithienyl
About This Item
Produtos recomendados
Ensaio
99%
p.e.
260 °C (lit.)
pf
32-33 °C (lit.)
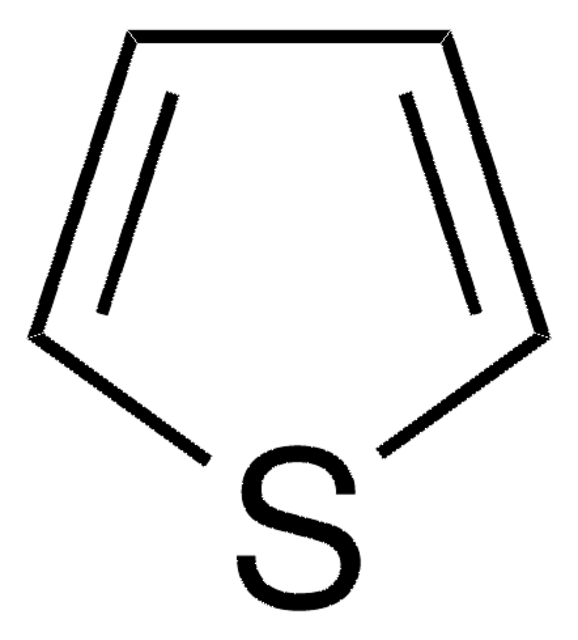
cadeia de caracteres SMILES
c1csc(c1)-c2cccs2
InChI
1S/C8H6S2/c1-3-7(9-5-1)8-4-2-6-10-8/h1-6H
chave InChI
OHZAHWOAMVVGEL-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
230.0 °F - closed cup
Ponto de fulgor (°C)
110 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Professor Rivnay (Northwestern University, USA) discusses using organic mixed conductors as an alternative to efficiently bridge the ionic world of biology with contemporary microelectronics.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)
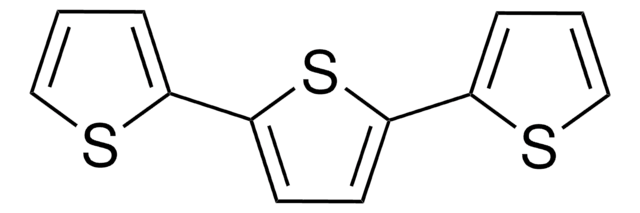
![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)