741027
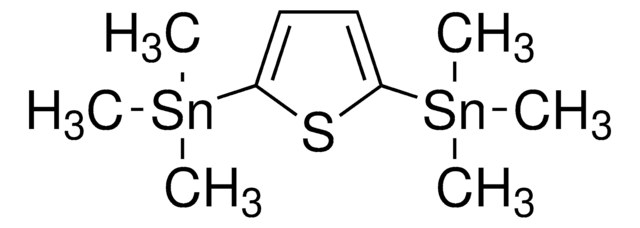
2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene
97%
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder or crystals
pf
127-132 °C
cadeia de caracteres SMILES
C[Sn](C)(C)c1cc2sc(cc2s1)[Sn](C)(C)C
InChI
1S/C6H2S2.6CH3.2Sn/c1-3-7-6-2-4-8-5(1)6;;;;;;;;/h1-2H;6*1H3;;
chave InChI
HDZULVYGCRXVNQ-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Aplicação
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 741027-1G | 4061826645109 |
| 741027-500MG | |
| 741027-5G | 4061832884448 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![1,1′-[4,8-Bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]](/deepweb/assets/sigmaaldrich/product/structures/611/912/a638a6fe-ca7b-4674-8023-df4c0921a9fd/640/a638a6fe-ca7b-4674-8023-df4c0921a9fd.png)


![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)
![Thieno[3,2-b]thiophene 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)