328774
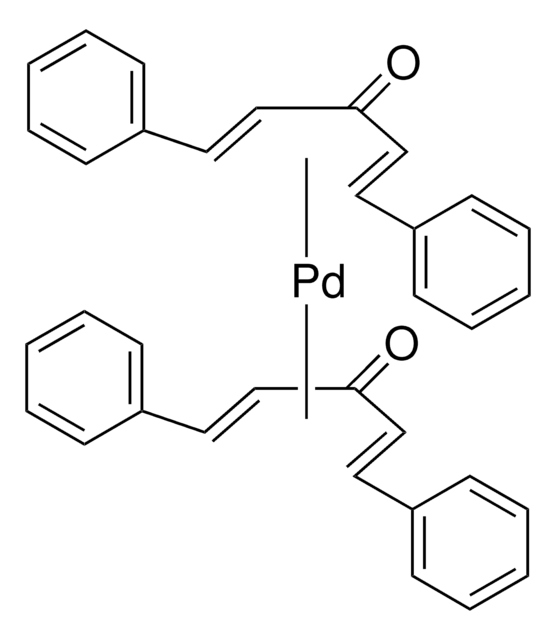
Tris(dibenzylideneacetone)dipalladium(0)
97%
Sinônimo(s):
Pd2dba3, Pd2(dba)3
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
powder
adequação da reação
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
pf
152-155 °C (lit.)
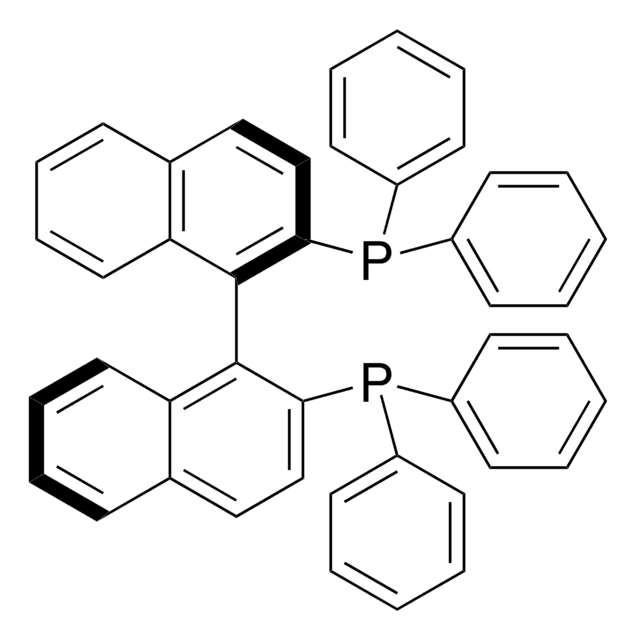
cadeia de caracteres SMILES
[Pd].[Pd].O=C(\C=C\c1ccccc1)/C=C/c2ccccc2.O=C(\C=C\c3ccccc3)/C=C/c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;;/h3*1-14H;;/b3*13-11+,14-12+;;
chave InChI
CYPYTURSJDMMMP-WVCUSYJESA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
For small scale and high throughput uses, product is also available as ChemBeads (919772)
Aplicação
- Application Guide for Palladium Catalyzed Cross-Coupling Reactions
- Synthesis of azepanes
- Synthesis of nanosized palladium phosphides upon interaction with white phosphorous
- Preparation of palladium triphenylphosphine carbonyl cluster complexes
- Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions
- Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynes
Reactant involved in:
- Catalyst for:
- Suzuki cross-coupling reactions
- PCN- and PCS-pincer palladium complex catalyzed tandem allylation
- Catalyst for Suzuki coupling of aryl chlorides (eq. 1)
- Catalyst for Heck coupling of aryl chlorides (eq. 2)
- Catalyst for arylation of ketones (eq. 3)
- Catalyst for Buchwald-Hartwig amination of aryl halides (eq. 4)
- Catalyst for fluorination of allylic chlorides (eq. 5)
- Catalyst for β-arylation of carboxylic esters (eq. 6)
- Catalyst for carbonylation of 1,1-dichloro-1-alkenes (eq. 7)
- Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides (eq. 8)
- Pd source for enantioselective Tsuji Allylations

Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 2 - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
JosiPhos CyPF-tBu and palladium give catalyst for alkoxylation of activated heteroaryl halides with primary, secondary, and tertiary alcohols
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 328774-1EA | |
| 328774-1G | 4061826717776 |
| 328774-500MG | 4061826717790 |
| 328774-100G | 4061833546635 |
| 328774-25G | 4061826717783 |
| 328774-500G | 4061833498965 |
| 328774-50G | 4061826717806 |
| 328774-5G | 4061826717813 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)