228931
Cerium(III) chloride heptahydrate
99.9% trace metals basis
Sinônimo(s):
Cerous chloride heptahydrate
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
Ensaio
99.9% trace metals basis
Formulário
crystals and lumps
adequação da reação
reagent type: catalyst
core: cerium
Impurezas
≤1500.0 ppm Trace Rare Earth Analysis
densidade
~3.94 g/mL at 25 °C (lit.)
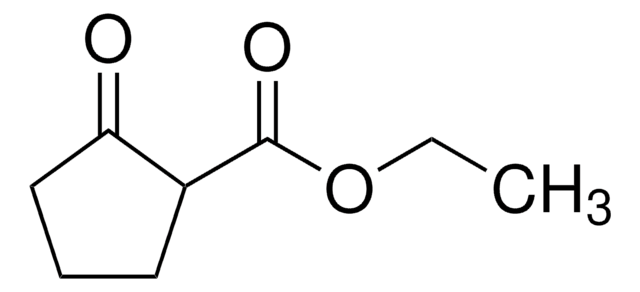
cadeia de caracteres SMILES
[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].Cl[Ce](Cl)Cl
InChI
1S/Ce.3ClH.7H2O/h;3*1H;7*1H2/q+3;;;;;;;;;;/p-3
chave InChI
KPZSTOVTJYRDIO-UHFFFAOYSA-K
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
- As a precursor to prepare cerium oxide nanoparticles for biomedical applications and photocatalytic degradation.
- As a solution to fabricate thin films of CeO2 on glass substrates by the spray pyrolysis process.
- As a dopant to fabricate ZnO and CeO2 nanocrystals for electrochemical sensing of H2O2 and photocatalytic degradation of Rhodamine B and Congo red dyes.
- As an additive to prepare corrosion-inhibiting formulations and coatings.
- To synthesize carbon nanofiber composites to fabricate high-temperature polymer electrolyte membrane fuel cell cathodes.
- As a support for the combination of cerium(III)chloride heptahydrate and sodium iodide supported on silica gel to promoteMichael-type additions. These catalysts are used to convert from indolesand nitroalkenes to 2-indolyl-1-nitroalkane derivatives in good yields.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The rare earth elements impact nearly everyone in the world. All of the people living in advanced technological countries and almost all those living in third world countries utilize the rare earths in their everyday living—the car that one drives (gasoline is refined from oil using rare earth catalysts and catalytic converters reduce the polluting emissions from the automotive exhaust), watching the news on TV (the red and green colors in TV screens), the telephones and computers we use to communicate (the permanent magnets in speakers and disc drives), just to name a few examples.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica