201146
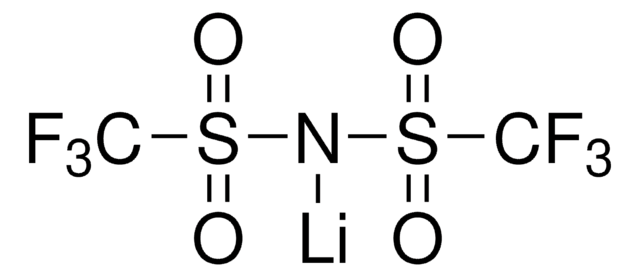
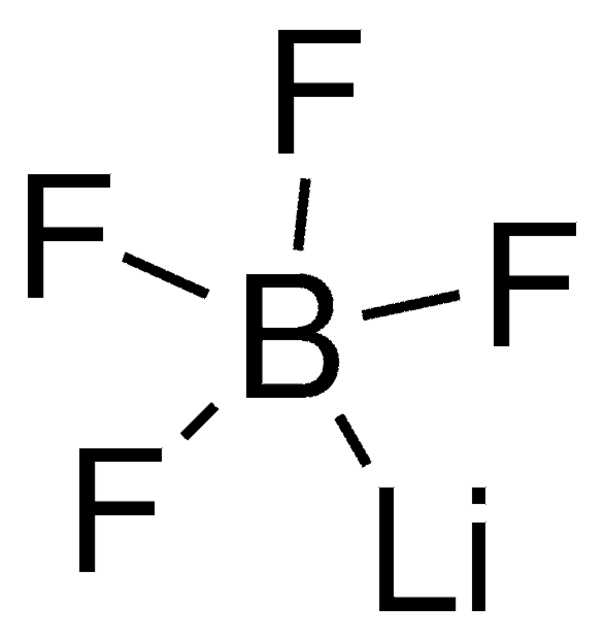
Lithium hexafluorophosphate
98%
Sinônimo(s):
Lithium phosphorus fluoride
About This Item
Produtos recomendados
grau
for analytical purposes
Nível de qualidade
Ensaio
98%
Formulário
powder
pf
200 °C (dec.) (lit.)
solubilidade
H2O: slightly soluble(lit.)
densidade
1.5 g/mL (lit.)
cadeia de caracteres SMILES
[Li+].F[P-](F)(F)(F)(F)F
InChI
1S/F6P.Li/c1-7(2,3,4,5)6;/q-1;+1
chave InChI
AXPLOJNSKRXQPA-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Características e benefícios
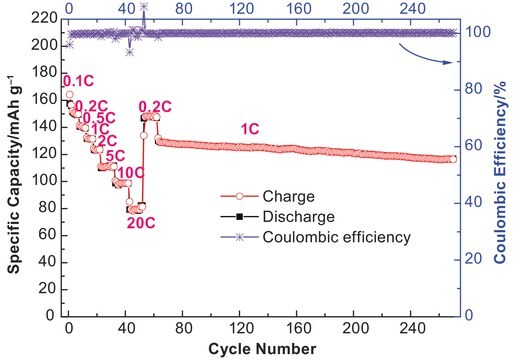
- It can form suitable SEI membranes in electrodes, especially in the cathode
- It can implement passivation for anode current collectors to prevent their dissolution
- Wide windows of electrical stability
- Excellent solubility and high conductivity in various solvents
- Environment-friendly
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1A - STOT RE 1 Inhalation
Órgãos-alvo
Bone,Teeth
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica