About This Item
Fórmula linear:
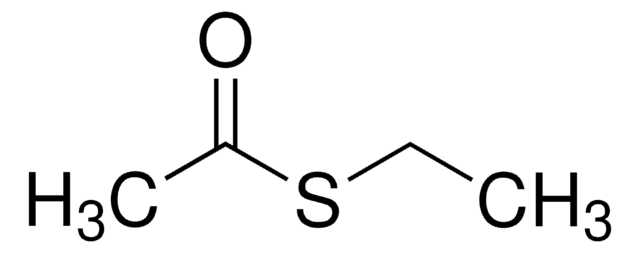
CH3COSC6H5
Número CAS:
Peso molecular:
152.21
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
Formulário
liquid
índice de refração
n20/D 1.57 (lit.)
p.e.
99-100 °C/6 mmHg (lit.)
densidade
1.124 g/mL at 25 °C (lit.)
grupo funcional
thioester
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CC(=O)Sc1ccccc1
InChI
1S/C8H8OS/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
chave InChI
WBISVCLTLBMTDS-UHFFFAOYSA-N
Categorias relacionadas
Aplicação
S-Phenyl thioacetate was used as a substrate to measure the esterase activity.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
174.2 °F - closed cup
Ponto de fulgor (°C)
79 °C - closed cup
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Yu Yuan et al.
Journal of the American Chemical Society, 131(15), 5432-5437 (2009-04-22)
Described herein is the chemical synthesis of the Cys(29)-Gly(77) glycopeptide domain (22) of erythropoietin. Our initial ligation strategy targeted a C --> N termini condensation between glycopeptide 3 and peptide 4. However, the reaction was hindered by the "unattainable" reactivity
K Lorentz et al.
Clinica chimica acta; international journal of clinical chemistry, 308(1-2), 69-78 (2001-06-20)
Arylesterase (EC 3.1.1.2) activity in serum was specifically measured using thiophenyl acetate in a mechanized assay at 37 degrees C with 4-bromophenylboronic acid as inhibitor of cholinesterase and hexacyanoferrate-III as indicator. The systematic development of a routine method, apparent limitations
Anita Bosak et al.
Molecules (Basel, Switzerland), 25(1) (2020-01-18)
Mammalian paraoxonase-1 hydrolyses a very broad spectrum of esters such as certain drugs and xenobiotics. The aim of this study was to determine whether carbamates influence the activity of recombinant PON1 (rePON1). Carbamates were selected having a variety of applications:
D I Draganov et al.
The Journal of biological chemistry, 275(43), 33435-33442 (2000-08-10)
The paraoxonase gene family contains at least three members: PON1, PON2, and PON3. The physiological roles of the corresponding gene products are still uncertain. Until recently, only the serum paraoxonase/arylesterase (PON1) had been purified and characterized. Here we report the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica