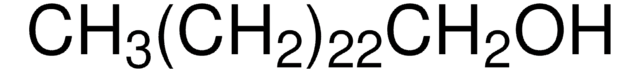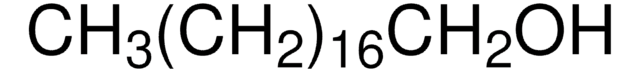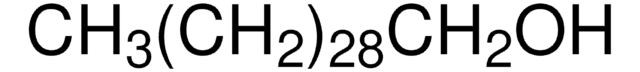169102
1-Docosanol
98%
Sinônimo(s):
Behenyl alcohol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
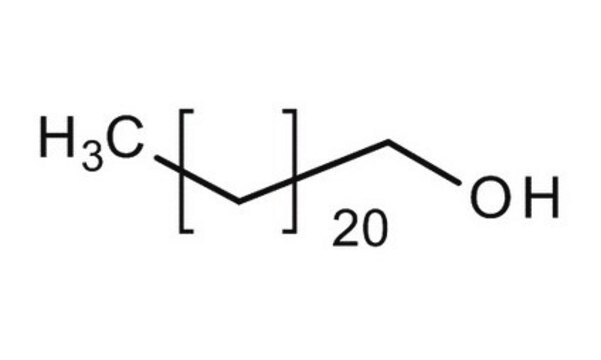
Fórmula linear:
CH3(CH2)21OH
Número CAS:
Peso molecular:
326.60
Beilstein:
1770470
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
p.e.
180 °C/0.22 mmHg (lit.)
pf
65-72 °C (lit.)
grupo funcional
hydroxyl
cadeia de caracteres SMILES
CCCCCCCCCCCCCCCCCCCCCCO
InChI
1S/C22H46O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23/h23H,2-22H2,1H3
chave InChI
NOPFSRXAKWQILS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
1-Docosanol inhibits replication of certain viruses (herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. It has been isolated from Clematis brevicaudata.
Aplicação
1-Docosanol was used in the synthesis of series of amphiphilic dendrimers with hydrophilic aliphatic polyether-type dendritic core and hydrophobic docosyl peripheries.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
nwg
Ponto de fulgor (°F)
410.0 °F
Ponto de fulgor (°C)
210 °C
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Ai-Mei Yang et al.
Zhong yao cai = Zhongyaocai = Journal of Chinese medicinal materials, 32(10), 1534-1537 (2010-02-02)
To study the chemical constituents from Clematis brevicaudata. The compounds were isolated by column chromatography and their structures were elucidated through spectroscopic analysis (NMR). Eight compounds were isolated and identified as: palmitic acid (1), 1-docosanol (2), pentacosanoic acid-2', 3'-dihydroxypropyl ester
Synthesis and self-assembly of amphiphilic dendrimers based on aliphatic polyether-type dendritic cores.
Cho B-K, et al.
Macromolecules, 37(11), 4227-4234 (2004)
Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex.
D H Katz et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825-10829 (1991-12-01)
This article reports that 1-docosanol, a 22-carbon-long saturated alcohol, exerts a substantial inhibitory effect on replication of certain viruses (e.g., herpes simplex virus and respiratory syncytial virus) within primary target cells in vitro. To study the basis for its viral
John F Marcelletti
Antiviral research, 56(2), 153-166 (2002-10-09)
Interactions between docosanol (n-docosanol, behenyl alcohol) and nucleoside or pyrophosphate analogs were investigated in vitro. The anti-HSV activity of acyclovir (ACV) was synergistically enhanced by treatment of cells with docosanol as judged by inhibition of progeny virus production and plaque
Clara L Shaw et al.
Journal of chemical ecology, 37(4), 329-339 (2011-03-23)
The uropygial secretions of some bird species contain volatile and semivolatile compounds that are hypothesized to serve as chemical signals. The abundance of secretion components varies with age and season, although these effects have not been investigated in many species.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica