136581
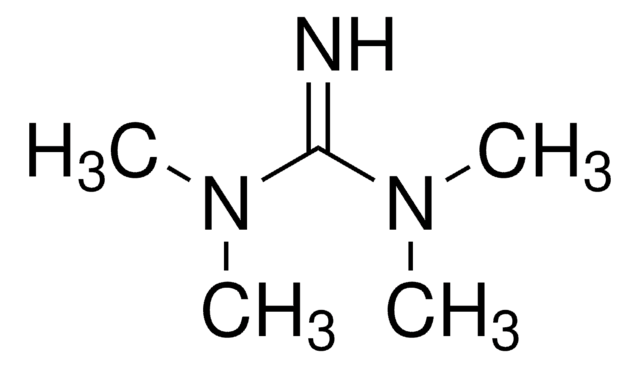
1,5-Diazabicyclo[4.3.0]non-5-ene
98%
Sinônimo(s):
DBN
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C7H12N2
Número CAS:
Peso molecular:
124.18
Beilstein:
2417
Número CE:
Número MDL:
Código UNSPSC:
12352005
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
liquid
índice de refração
n20/D 1.519 (lit.)
p.e.
95-98 °C/7.5 mmHg (lit.)
densidade
1.005 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
C1CN=C2CCCN2C1
InChI
1S/C7H12N2/c1-3-7-8-4-2-6-9(7)5-1/h1-6H2
chave InChI
SGUVLZREKBPKCE-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
DBN is a strong base widely used as a catalyst in organic synthesis to facilitate Michael additions and aldol condensations.
Aplicação
1,5-Diazabicyclo[4.3.0]non-5-ene (DBN) can be used as:
- A reagent for the synthesis of β-carbolines from tetrahydro-β-carbolines via dehydrogenative/decarboxylative aromatization.
- A nucleophilic organocatalyst in the regioselective C-acylation of pyrroles and indoles by Friedel−Crafts acylation reaction.
- A superbase in the formulation of a ternary liquid-liquid phase changing system, along with hexadecane and hexanol, to capture hydrogen sulfide gas.
- A base for the preparation of 1H-quinazoline-2,4-diones from 2-aminobenzonitriles using supercritical carbon dioxide as a reactant and a solvent.
- A catalyst for the synthesis of benzothiazolones by the reaction between 2-aminothiophenols and CO2 by cyclocarbonylation reaction via C-S bond formation.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Skin Corr. 1B
Código de classe de armazenamento
8A - Combustible corrosive hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
201.2 °F - closed cup
Ponto de fulgor (°C)
94 °C - closed cup
Equipamento de proteção individual
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Arinze Akutekwe et al.
IET systems biology, 9(6), 294-302 (2015-11-19)
Accurate and reliable modelling of protein-protein interaction networks for complex diseases such as colorectal cancer can help better understand mechanism of diseases and potentially discover new drugs. Different machine learning methods such as empirical mode decomposition combined with least square
Organic Base-Catalyzed C-S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones.
Gao Xiang, et al.
Catalysts, 8(7), 271-271 (2018)
Organic base-promoted efficient dehydrogenative/decarboxylative aromatization of tetrahydro-βcarbolines into β-carbolines under air.
Zhao Z, et al.
Tetrahedron Letters, 60(11), 800-804 (2019)
The simple solvent-free synthesis of 1H-quinazoline-2, 4-diones using supercritical carbon dioxide and catalytic amount of base
Mizuno T, et al.
Tetrahedron Letters, 45(38), 7073-7075 (2004)
Phase-Change Reversible Absorption of Hydrogen Sulfide by the Superbase 1, 5-Diazabicyclo [4.3. 0] non-5-ene in Organic Solvents.
Xu Z, et al.
Industrial & Engineering Chemistry Research, 58(4), 1701-1710 (2019)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 136581-5G | 4061837670244 |
| 136581-100G | 4061838731357 |
| 136581-25G | 4061837670237 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica![1,8-Diazabiciclo[5,4,0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)

![1,8-Diazabicyclo[5.4.0]undec-7-ene for synthesis](/deepweb/assets/sigmaaldrich/product/images/219/652/f12d7266-2d82-4869-9d8d-919b0f68de68/640/f12d7266-2d82-4869-9d8d-919b0f68de68.jpg)