About This Item
Fórmula empírica (Notação de Hill):
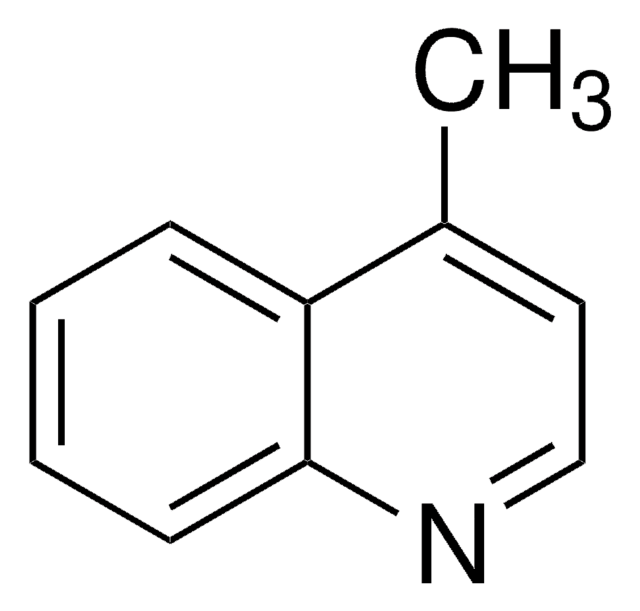
C10H9N
Número CAS:
Peso molecular:
143.19
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
pb
251 °C (lit.)
pf
63-65 °C (lit.)
cadeia de caracteres SMILES
Cc1cc2ccccc2cn1
InChI
1S/C10H9N/c1-8-6-9-4-2-3-5-10(9)7-11-8/h2-7H,1H3
chave InChI
FVVXWRGARUACNW-UHFFFAOYSA-N
Informações sobre genes
human ... CYP1A2(1544)
Descrição geral
The metabolites of 3-methylisoquinoline were separated by adsorption and reversed-phase high-performance liquid chromatography (HPLC).
Aplicação
3-Methylisoquinoline was used to prepare 3-aminoisoquinoline.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
The Preparation of 3-Aminoisoquinoline and Related Compounds1.
Journal of the American Chemical Society, 73(2), 688-689 (1951)
C Stubley et al.
Journal of chromatography, 177(2), 313-322 (1979-09-21)
Adsorption and reversed-phase high-performance liquid chromatography (HPLC) have been successfully used to separate metabolites from the parent heterocycles (isoquinoline, 3-methylisoquinoline, phthalazine, quinazoline, quinoxaline and cinnoline). Retention data are reported. The metabolites, hydroxyazanaphthalenes, which arise as a result of aldehyde oxidase
Elisabetta Muntoni et al.
Pharmaceutics, 11(2) (2019-02-06)
Glioblastoma is the most common and invasive primary tumor of the central nervous system and normally has a negative prognosis. Biodistribution in healthy animal models is an important preliminary study aimed at investigating the efficacy of chemotherapy, as it is
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Kunal Roy et al.
European journal of medicinal chemistry, 44(5), 1941-1951 (2008-12-27)
A series of naphthalene and non-naphthalene derivatives (n=42) having cytochrome P450 2A6 and 2A5 inhibitory activities, reported by Rahnasto et al., were subjected to QSAR and QAAR studies. The analyses were performed using electronic, spatial, shape and thermodynamic descriptors to
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica