About This Item
Fórmula linear:
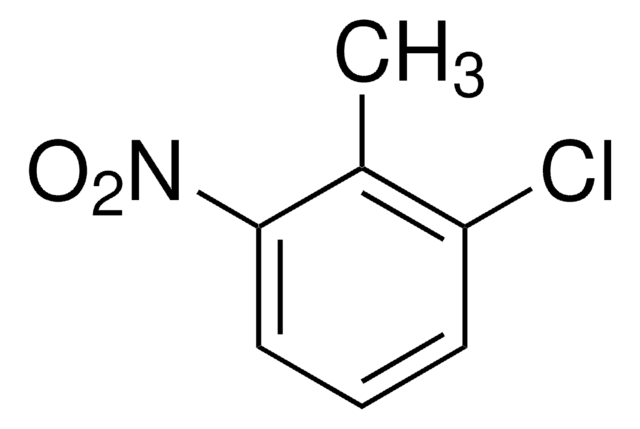
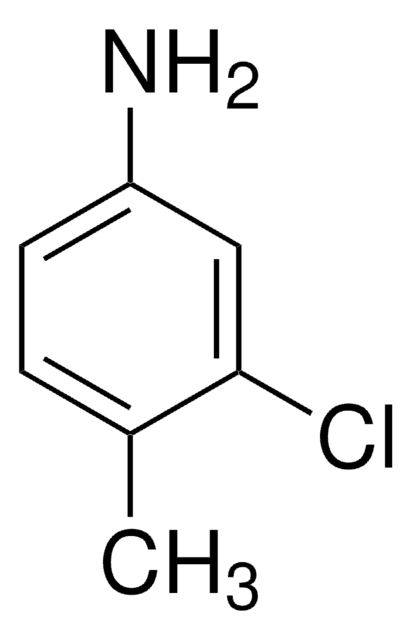
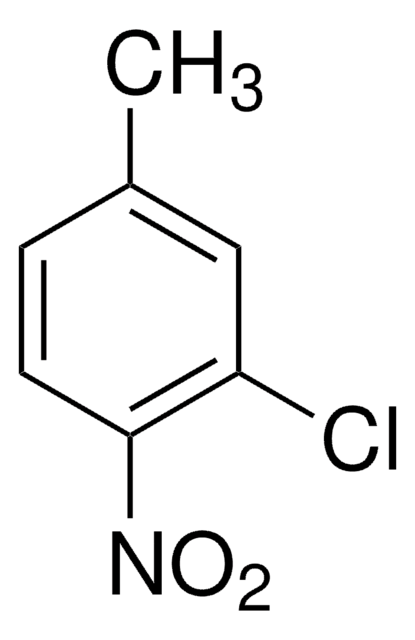
CH3C6H3(NO2)Cl
Número CAS:
Peso molecular:
171.58
Beilstein:
2046651
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
forma
solid
pb
239-240 °C/718 mmHg (lit.)
pf
34-38 °C (lit.)
grupo funcional
chloro
nitro
cadeia de caracteres SMILES
Cc1ccc(Cl)cc1[N+]([O-])=O
InChI
1S/C7H6ClNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H,1H3
chave InChI
SQFLFRQWPBEDHM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
4-Chloro-2-nitrotoluene can be used in the synthesis of indigo dye.
Ações bioquímicas/fisiológicas
4-Chloro-2-nitrotoluene is the starting reagent for synthesis of tricyclic indole-2-carboxylic acids, a potential NMDA-glycine antagonists.
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
248.0 °F - closed cup
Ponto de fulgor (°C)
120 °C - closed cup
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Thanasis Karapanayiotis et al.
The Analyst, 129(7), 613-618 (2004-06-24)
The Raman and electron impact mass spectra of synthetic indigo and its four 6,6'-dihalogeno analogues are reported and discussed. The influence of varying the halogen on these Raman spectra is considered. Particular emphasis is laid on distinguishing indigo from 6,6'-dibromoindigo
S Katayama et al.
The Journal of organic chemistry, 66(10), 3474-3483 (2001-05-12)
The practical synthesis of a series of tricyclic indole-2-carboxylic acids, 7-chloro-3-arylaminocarbonylmethyl-1,3,4,5-tetrahydrobenz[cd]indole-2-carboxylic acids, as a new class of potent NMDA-glycine antagonists is described. The synthetic route to the key intermediate 12a comprises a regioselective iodination of 4-chloro-2-nitrotoluene, modified Reissert indole synthesis
D de Almeida Azevedo et al.
Journal of chromatography. A, 879(1), 13-26 (2000-06-28)
Gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-atmospheric pressure chemical ionization mass spectrometry (LC-APCI-MS) were optimized and applied for the trace-level determination of 42 priority pesticides and 33 priority organic pollutants from European Union Directive EC 76/464. First, off-line solid-phase extraction
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica