914134
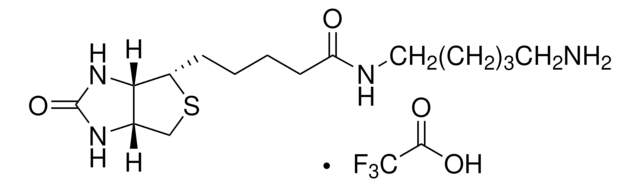
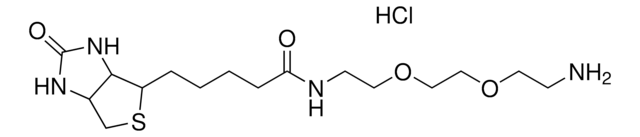
5-(Biotinamido)pentylamine TFA Salt
≥95%
Synonyme(s) :
N-(5-Aminopentyl)-5-((3aS,4S,6aR)-2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pent, Biotin cadaverine TFA, Biotin-DAPe TFA
About This Item
Produits recommandés
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Autres remarques
Convergent synthesis of trifunctional molecules by three sequential azido-type-selective cycloadditions
Locked by Design: A Conformationally Constrained Transglutaminase Tag Enables Efficient Site-Specific Conjugation
Synthesis of Novel Phosphonic-Type Activity-Based Probes for Neutrophil Serine Proteases and Their Application in Spleen Lysates of Different Organisms
Produit(s) apparenté(s)
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Désolés, nous n'avons pas de COA pour ce produit disponible en ligne pour le moment.
Si vous avez besoin d'assistance, veuillez contacter Service Clients
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique